Contribution of impaired myocardial insulin signaling to mitochondrial dysfunction and oxidative stress in the heart
- PMID: 19237663
- PMCID: PMC2739097
- DOI: 10.1161/CIRCULATIONAHA.108.792101
Contribution of impaired myocardial insulin signaling to mitochondrial dysfunction and oxidative stress in the heart
Abstract
Background: Diabetes-associated cardiac dysfunction is associated with mitochondrial dysfunction and oxidative stress, which may contribute to left ventricular dysfunction. The contribution of altered myocardial insulin action, independent of associated changes in systemic metabolism, is incompletely understood. The present study tested the hypothesis that perinatal loss of insulin signaling in the heart impairs mitochondrial function.
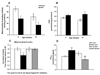
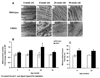
Methods and results: In 8-week-old mice with cardiomyocyte deletion of insulin receptors (CIRKO), inotropic reserves were reduced, and mitochondria manifested respiratory defects for pyruvate that was associated with proportionate reductions in catalytic subunits of pyruvate dehydrogenase. Progressive age-dependent defects in oxygen consumption and ATP synthesis with the substrate glutamate and the fatty acid derivative palmitoyl-carnitine were observed. Mitochondria also were uncoupled when exposed to palmitoyl-carnitine, in part as a result of increased reactive oxygen species production and oxidative stress. Although proteomic and genomic approaches revealed a reduction in subsets of genes and proteins related to oxidative phosphorylation, no reductions in maximal activities of mitochondrial electron transport chain complexes were found. However, a disproportionate reduction in tricarboxylic acid cycle and fatty acid oxidation proteins in mitochondria suggests that defects in fatty acid and pyruvate metabolism and tricarboxylic acid flux may explain the mitochondrial dysfunction observed.
Conclusions: Impaired myocardial insulin signaling promotes oxidative stress and mitochondrial uncoupling, which, together with reduced tricarboxylic acid and fatty acid oxidative capacity, impairs mitochondrial energetics. This study identifies specific contributions of impaired insulin action to mitochondrial dysfunction in the heart.
Conflict of interest statement
(None).
Figures


References
-
- Boudina S, Abel ED. Mitochondrial uncoupling: a key contributor to reduced cardiac efficiency in diabetes. Physiology (Bethesda) 2006;21:250–258. - PubMed
-
- Boudina S, Abel ED. Diabetic cardiomyopathy revisited. Circulation. 2007;115:3213–3223. - PubMed
-
- Boudina S, Sena S, O'Neill BT, Tathireddy P, Young ME, Abel ED. Reduced mitochondrial oxidative capacity and increased mitochondrial uncoupling impair myocardial energetics in obesity. Circulation. 2005;112:2686–2695. - PubMed
-
- Boudina S, Sena S, Theobald H, Sheng X, Wright JJ, Hu XX, Aziz S, Johnson JI, Bugger H, Zaha VG, Abel ED. Mitochondrial energetics in the heart in obesity-related diabetes: direct evidence for increased uncoupled respiration and activation of uncoupling proteins. Diabetes. 2007;56:2457–2466. - PubMed
-
- Bugger H, Boudina S, Hu XX, Tuinei J, Zaha VG, Theobald HA, Yun UJ, McQueen AP, Wayment B, Litwin SE, Abel ED. Type 1 Diabetic Akita Mouse Hearts are Insulin Sensitive but Manifest Structurally Abnormal Mitochondria that Remain Coupled Despite Increased Uncoupling Protein 3. Diabetes. 2008;57:2924–2932. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

