Long-term cardiac-targeted RNA interference for the treatment of heart failure restores cardiac function and reduces pathological hypertrophy
- PMID: 19237664
- PMCID: PMC4298485
- DOI: 10.1161/CIRCULATIONAHA.108.783852
Long-term cardiac-targeted RNA interference for the treatment of heart failure restores cardiac function and reduces pathological hypertrophy
Abstract
Background: RNA interference (RNAi) has the potential to be a novel therapeutic strategy in diverse areas of medicine. Here, we report on targeted RNAi for the treatment of heart failure, an important disorder in humans that results from multiple causes. Successful treatment of heart failure is demonstrated in a rat model of transaortic banding by RNAi targeting of phospholamban, a key regulator of cardiac Ca(2+) homeostasis. Whereas gene therapy rests on recombinant protein expression as its basic principle, RNAi therapy uses regulatory RNAs to achieve its effect.
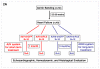
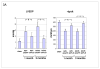
Methods and results: We describe structural requirements to obtain high RNAi activity from adenoviral and adeno-associated virus (AAV9) vectors and show that an adenoviral short hairpin RNA vector (AdV-shRNA) silenced phospholamban in cardiomyocytes (primary neonatal rat cardiomyocytes) and improved hemodynamics in heart-failure rats 1 month after aortic root injection. For simplified long-term therapy, we developed a dimeric cardiotropic adeno-associated virus vector (rAAV9-shPLB) to deliver RNAi activity to the heart via intravenous injection. Cardiac phospholamban protein was reduced to 25%, and suppression of sacroplasmic reticulum Ca(2+) ATPase in the HF groups was rescued. In contrast to traditional vectors, rAAV9 showed high affinity for myocardium but low affinity for liver and other organs. rAAV9-shPLB therapy restored diastolic (left ventricular end-diastolic pressure, dp/dt(min), and tau) and systolic (fractional shortening) functional parameters to normal ranges. The massive cardiac dilation was normalized, and cardiac hypertrophy, cardiomyocyte diameter, and cardiac fibrosis were reduced significantly. Importantly, no evidence was found of microRNA deregulation or hepatotoxicity during these RNAi therapies.
Conclusions: Our data show for the first time the high efficacy of an RNAi therapeutic strategy in a cardiac disease.
Figures














References
-
- Schmidt U, Hajjar RJ, Kim CS, Lebeche D, Doye A, Gwathmey J. Human heart failure: cAMP stimulation of SR Ca2+-ATPase activity and phosphorylation level of phospholamban. Am J Physiol. 1999;277:H474–480. - PubMed
-
- MacLennan D, Kranias E. Phospholamban: A crucial regulator of cardiac contractility. Nature Reviews Molecular Cell Biology. 2003;4:566–576. - PubMed
-
- Hoshijima M, Ikeda Y, Iwanaga Y, Minamisawa S, Date M, Gu Y, Iwatate M, Li M, Wang L, Wilson J, Wang Y, Ross J, Chien K. Chronic suppression of heart-failure progression by a pseudophosphorylated mutant of phospholamban via in vivo cardiac rAAV gene delivery. Nature Medicine. 2002;8:864–871. - PubMed
-
- Iwanaga Y, Hoshijima M, Gu Y, Iwatate M, Dieterle T, Ikeda Y, Date MO, Chrast J, Matsuzaki M, Peterson KL, Chien KR, Ross J., Jr. Chronic phospholamban inhibition prevents progressive cardiac dysfunction and pathological remodeling after infarction in rats. J Clin Invest. 2004;113:727–736. - PMC - PubMed
-
- Eizema K, Fechner H, Bezstarosti K, Schneider-Rasp S, van der Laarse A, Wang H, Schultheiss H-P, Poller W, Lamers J. Adenovirus-based phospholamban-antisense-mRNA expression as a novel approach to improve cardiac contractile dysfunction - Comparison of a constitutive viral versus an endothelin-1-responsive cardiac promoter. Circulation. 2000;101:2193–2199. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

