Sex-lethal facilitates the transition from germline stem cell to committed daughter cell in the Drosophila ovary
- PMID: 19237687
- PMCID: PMC2674811
- DOI: 10.1534/genetics.109.100693
Sex-lethal facilitates the transition from germline stem cell to committed daughter cell in the Drosophila ovary
Abstract
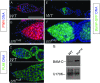
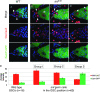
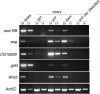
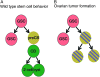
In Drosophila, the female-specific SEX-LETHAL (SXL) protein is required for oogenesis, but how Sxl interfaces with the genetic circuitry controlling oogenesis remains unknown. Here we use an allele of sans fille (snf) that specifically eliminates SXL protein in germ cells to carry out a detailed genetic and cell biological analysis of the resulting ovarian tumor phenotype. We find that tumor growth requires both Cyclin B and zero population growth, demonstrating that these mutant cells retain at least some of the essential growth-control mechanisms used by wild-type germ cells. Using a series of molecular markers, we establish that while the tumor often contains at least one apparently bona fide germline stem cell, the majority of cells exhibit an intermediate fate between a stem cell and its daughter cell fated to differentiate. In addition, snf tumors misexpress a select group of testis-enriched markers, which, remarkably, are also misexpressed in ovarian tumors that arise from the loss of bag of marbles (bam). Results of genetic epistasis experiments further reveal that bam's differentiation-promoting function depends on Sxl. Together these data demonstrate a novel role for Sxl in the lineage progression from stem cell to committed daughter cell and suggest a model in which Sxl partners with bam to facilitate this transition.
Figures
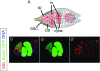
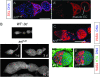
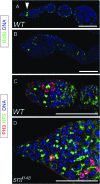
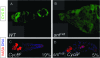

References
-
- Bopp, D., L. R. Bell, T. W. Cline and P. Schedl, 1991. Developmental distribution of female-specific SEX-LETHAL proteins in Drosophila melanogaster. Genes Dev. 5 403–415. - PubMed
-
- Bopp, D., J. I. Horabin, R. A. Lersch, T. W. Cline and P. Schedl, 1993. Expression of the Sex-lethal gene is controlled at multiple levels during Drosophila oogenesis. Development 118 797–812. - PubMed
-
- Casanueva, M. O., and E. L. Ferguson, 2004. Germline stem cell number in the Drosophila ovary is regulated by redundant mechanisms that control DPP signaling. Development 131 1881–1890. - PubMed
-
- Casper, A., and M. Van Doren, 2006. The control of sexual identity in the Drosophila germline. Development 133 2783–2791. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

