Targeting the intratumoral dendritic cells by the oncolytic adenoviral vaccine expressing RANTES elicits potent antitumor immunity
- PMID: 19238013
- PMCID: PMC4146345
- DOI: 10.1097/CJI.0b013e318193d31e
Targeting the intratumoral dendritic cells by the oncolytic adenoviral vaccine expressing RANTES elicits potent antitumor immunity
Abstract
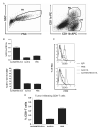
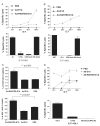
Dendritic cells (DCs) are professional antigen (Ag)-presenting cells capable of inducing immune responses to tumor Ags and, therefore, play a central role in the induction of antitumor immunity. There is a large amount of evidence, however, about paucity of tumor-associated DCs and that DCs' immunogenic functions are suppressed in a tumor environment. Here we describe a potent in situ vaccine targeting tumoral DCs in vivo. This vaccine comprised of an oncolytic adenovirus expressing RANTES (regulated upon activation, normally T expressed, and presumably secreted) (Ad-RANTES-E1A), enhanced tumor infiltration, and maturation of Ag-presenting cells in vivo. In this study, we show that intratumoral vaccinations with Ad-RANTES-E1A induced significant primary tumor growth regression and blocked metastasis formation in JC and E.G-7 murine tumor models. This vaccine recruited DCs, macrophages, natural killer cells, and CD8+ T cells to the tumor site, and thus enhanced Ag-specific cytotoxic T lymphocyte responses and natural killer cell responses. DCs purified from the Ad-RANTES-E1A-treated E.G-7 tumors secreted significantly higher levels of interferon-gamma and interleukin-12, as compared with control groups and more efficiently enhanced CD8+ T-cell response. This in situ immunization strategy could be a potent antitumor immunotherapy approach for aggressive established tumors.
Figures




Similar articles
-
Attraction and activation of dendritic cells at the site of tumor elicits potent antitumor immunity.Mol Ther. 2009 Sep;17(9):1626-36. doi: 10.1038/mt.2009.111. Epub 2009 Jun 16. Mol Ther. 2009. PMID: 19532135 Free PMC article.
-
The boosting effect of co-transduction with cytokine genes on cancer vaccine therapy using genetically modified dendritic cells expressing tumor-associated antigen.Int J Oncol. 2006 Apr;28(4):947-53. Int J Oncol. 2006. PMID: 16525645
-
Dual antigen target-based immunotherapy for prostate cancer eliminates the growth of established tumors in mice.Immunotherapy. 2011 Jun;3(6):735-46. doi: 10.2217/imt.11.59. Immunotherapy. 2011. PMID: 21668311
-
The Journey of in vivo Virus Engineered Dendritic Cells From Bench to Bedside: A Bumpy Road.Front Immunol. 2018 Sep 11;9:2052. doi: 10.3389/fimmu.2018.02052. eCollection 2018. Front Immunol. 2018. PMID: 30254636 Free PMC article. Review.
-
Virology- and immunology-based gene therapy for cancer.Cancer Immunol Immunother. 2006 Nov;55(11):1420-5. doi: 10.1007/s00262-006-0173-3. Epub 2006 May 12. Cancer Immunol Immunother. 2006. PMID: 16691360 Free PMC article. Review.
Cited by
-
Reduced tumourigenicity of EG7 after RANTES gene transfer and the underlying mechanism.Arch Med Sci. 2010 Dec;6(6):829-36. doi: 10.5114/aoms.2010.19287. Epub 2010 Dec 29. Arch Med Sci. 2010. PMID: 22427753 Free PMC article.
-
Oncolytic Viruses for Cancer Therapy: Overcoming the Obstacles.Viruses. 2010 Jan;2(1):78-106. doi: 10.3390/v2010078. Viruses. 2010. PMID: 20543907 Free PMC article.
-
Expression of FMS-like tyrosine kinase 3 ligand by oncolytic herpes simplex virus type I prolongs survival in mice bearing established syngeneic intracranial malignant glioma.Neurosurgery. 2012 Sep;71(3):741-8; discussion 748. doi: 10.1227/NEU.0b013e318260fd73. Neurosurgery. 2012. PMID: 22653387 Free PMC article.
-
Biomimetic Nucleic Acid Drug Delivery Systems for Relieving Tumor Immunosuppressive Microenvironment.Pharmaceutics. 2024 Aug 1;16(8):1028. doi: 10.3390/pharmaceutics16081028. Pharmaceutics. 2024. PMID: 39204373 Free PMC article. Review.
-
An engineered oncolytic virus expressing PD-L1 inhibitors activates tumor neoantigen-specific T cell responses.Nat Commun. 2020 Mar 13;11(1):1395. doi: 10.1038/s41467-020-15229-5. Nat Commun. 2020. PMID: 32170083 Free PMC article.
References
-
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245. - PubMed
-
- Steinman RM. The control of immunity and tolerance by dendritic cell. Pathol Biol (Paris) 2003;51:59. - PubMed
-
- Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2:151. - PubMed
-
- Godelaine D, Carrasco J, Lucas S, et al. Polyclonal CTL responses observed in melanoma patients vaccinated with dendritic cells pulsed with a MAGE-3A1 peptide. J Immunol. 2003;171:4893. - PubMed
-
- Kugler A, Stuhler G, Walden P, et al. Regression of human metastatic renal cell carcinoma after vaccination with tumor cell-dendritic cell hybrids. Nat Med. 2000;6:332. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

