Localisation and function of the endocannabinoid system in the human ovary
- PMID: 19238202
- PMCID: PMC2640464
- DOI: 10.1371/journal.pone.0004579
Localisation and function of the endocannabinoid system in the human ovary
Abstract
Background: Although anandamide (AEA) had been measured in human follicular fluid and is suggested to play a role in ovarian follicle and oocyte maturity, its exact source and role in the human ovary remains unclear.
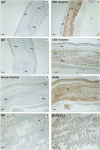
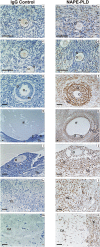
Methods and findings: Immunohistochemical examination of normal human ovaries indicated that the endocannabinoid system was present and widely expressed in the ovarian medulla and cortex with more intense cannabinoid receptor 2 (CB2) than CB1 immunoreactivity in the granulosa cells of primordial, primary, secondary, tertiary follicles, corpus luteum and corpus albicans. The enzymes, fatty acid amide hydrolase (FAAH) and N-acyclphosphatidylethanolamine-phospholipase D (NAPE-PLD), were only found in growing secondary and tertiary follicles and corpora lutea and albicantes. The follicular fluid (FF) AEA concentrations of 260 FF samples, taken from 37 infertile women undergoing controlled ovarian hyperstimulation for in vitro fertilisation and intracytoplasmic sperm injection with embryo transfer, were correlated with ovarian follicle size (P = 0.03). Significantly higher FF AEA concentrations were also observed in mature follicles (1.43+/-0.04 nM; mean+/-SEM) compared to immature follicles (1.26+/-0.06 nM), P = 0.0142 and from follicles containing morphologically assessed mature oocytes (1.56+/-0.11 nM) compared to that containing immature oocytes (0.99+/-0.09 nM), P = 0.0011. ROC analysis indicated that a FF AEA level of 1.09 nM could discriminate between mature and immature oocytes with 72.2% sensitivity and 77.14% specificity, whilst plasma AEA levels and FF AEA levels on oocyte retrieval day were not significantly different (P = 0.23).
Conclusions: These data suggest that AEA is produced in the ovary, is under hormonal control and plays a role in folliculogenesis, preovulatory follicle maturation, oocyte maturity and ovulation.
Conflict of interest statement
Figures




References
-
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. - PubMed
-
- Childers SR, Breivogel CS. Cannabis and endogenous cannabinoid systems. Drug and Alcohol Dependence. 1998;51:173–187. - PubMed
-
- Habayeb OM, Bell SC, Konje JC. Endogenous cannabinoids: Metabolism and their role in reproduction. Life Sciences. 2002;70:1963–1977. - PubMed
-
- Piomelli D, Giuffrida A, Calignano A, Rodriguez de Fonseca F. The endocannabinoid system as a target for therapeutic drugs. Trends in Pharmacological Sciences. 2000;21:218–224. - PubMed
-
- Galiegue S, Mary S, Marchand J, Dussossoy D, Carriere D, et al. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. European Journal of Biochemistry. 1995;232:54–61. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

