Mood, side-effects and smoking outcomes among persons with and without probable lifetime depression taking varenicline
- PMID: 19238488
- PMCID: PMC2669860
- DOI: 10.1007/s11606-009-0926-8
Mood, side-effects and smoking outcomes among persons with and without probable lifetime depression taking varenicline
Erratum in
- J Gen Intern Med. 2009 Oct;24(10):1173
Abstract
Background: Varenicline may be associated with greater mood disturbance and side-effects among smokers with psychiatric history, but empirical evidence is limited. Differential treatment effectiveness by psychiatric history may also exist.
Objective: To compare mood, prevalence and intensity of treatment side-effects, and abstinence among people with a probable history of major depression (DH+) or not (DH-) who took varenicline and received behavioral smoking cessation treatment.
Design: Smokers participated in a randomized behavioral intervention effectiveness trial. Treatment side-effects and outcomes were compared between DH+ and DH- participants (n = 1,117) at 21 [corrected] days and 3 months after the target quit date.
Participants: Smokers recruited from a large regional health plan.
Measurements: Change in stress and depression scores, prevalence and intensity of treatment side-effects, and abstinence rates.
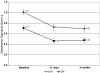
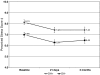
Results: All side-effects averaged moderate intensity or less and were similar across DH groups, except DH+'s endorsed slightly worse confusion, nausea (adjusted P = 0.04) and trouble sleeping (adjusted P = 0.008) at 21 days. Depression and stress scores declined in both DH groups and an equal proportion of each evidenced new/worsening depressive symptoms. Despite few differences in symptom intensity, more DH+ participants reported recent tension/agitation, irritability/anger, confusion, and depression at 21 days (adjusted P < 0.05), and depression and anxiety (adjusted P < 0.01) at three months. Nonsmoking rates did not differ by DH group at follow-up.
Conclusion: While some group differences were noted, DH+ smokers did not report qualitatively worse neuropsychiatric symptoms, more new/worsening mood disturbance, or differential abstinence rates compared to DH- smokers.
Trial registration: ClinicalTrials.gov NCT00301145.
Figures
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

