Molecular architecture of myelinated peripheral nerves is supported by calorie restriction with aging
- PMID: 19239416
- PMCID: PMC2715941
- DOI: 10.1111/j.1474-9726.2009.00460.x
Molecular architecture of myelinated peripheral nerves is supported by calorie restriction with aging
Abstract
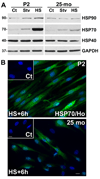
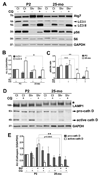
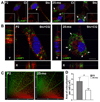
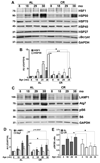
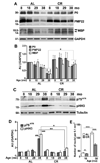
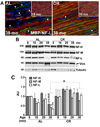
Peripheral nerves from aged animals exhibit features of degeneration, including marked fiber loss, morphological irregularities in myelinated axons and notable reduction in the expression of myelin proteins. To investigate how protein homeostatic mechanisms change with age within the peripheral nervous system, we isolated Schwann cells from the sciatic nerves of young and old rats. The responsiveness of cells from aged nerves to stress stimuli is weakened, which in part may account for the observed age-associated alterations in glial and axonal proteins in vivo. Although calorie restriction is known to slow the aging process in the central nervous system, its influence on peripheral nerves has not been investigated in detail. To determine if dietary restriction is beneficial for peripheral nerve health and glial function, we studied sciatic nerves from rats of four distinct ages (8, 18, 29 and 38 months) kept on an ad libitum (AL) or a 40% calorie restricted diet. Age-associated reduction in the expression of the major myelin proteins and widening of the nodes of Ranvier are attenuated by the dietary intervention, which is paralleled with the maintenance of a differentiated Schwann cell phenotype. The improvements in nerve architecture with diet restriction, in part, are underlined by sustained expression of protein chaperones and markers of the autophagy-lysosomal pathway. Together, the in vitro and in vivo results suggest that there might be an age-limit by which dietary intervention needs to be initiated to elicit a beneficial response on peripheral nerve health.
Figures

References
-
- Adinolfi AM, Yamuy J, Morales FR, Chase MH. Segmental demyelination in peripheral nerves of old cats. Neurobiol Aging. 1991;12:175–179. - PubMed
-
- Amici SA, Dunn WA, Jr, Notterpek L. Developmental abnormalities in the nerves of peripheral myelin protein 22-deficient mice. J Neurosci Res. 2007;85:238–249. - PubMed
-
- Aquino DA, Lopez C, Farooq M. Antisense oligonucleotide to the 70-kDa heat shock cognate protein inhibits synthesis of myelin basic protein. Neurochem Res. 1996;21:417–422. - PubMed
-
- Benz I, Beck W, Kraas W, Stoll D, Jung G, Kohlhardt M. Two types of modified cardiac Na+ channels after cytosolic interventions at the alpha-subunit capable of removing Na+ inactivation. Eur Biophys J. 1997;25:189–200. - PubMed
-
- Bergamini E, Cavallini G, Donati A, Gori Z. The anti-ageing effects of caloric restriction may involve stimulation of macroautophagy and lysosomal degradation, and can be intensified pharmacologically. Biomed Pharmacother. 2003;57:203–208. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

