The many roles of FAS receptor signaling in the immune system
- PMID: 19239902
- PMCID: PMC2956119
- DOI: 10.1016/j.immuni.2009.01.001
The many roles of FAS receptor signaling in the immune system
Abstract
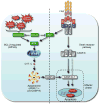
FAS belongs to the subgroup of the tumor necrosis factor receptor (TNF-R) family that contains an intracellular "death domain" and triggers apoptosis. Its physiological ligand FASL is a member of the TNF cytokine family. Studies with mutant mice and cells from human patients have shown that FAS plays critical roles in the immune system, including the killing of pathogen-infected cells and the death of obsolete and potentially dangerous lymphocytes. Fas thereby functions as a guardian against autoimmunity and tumor development. FAS triggers apoptosis through FADD-mediated recruitment and activation of caspase-8. In certain cells such as hepatocytes, albeit not lymphocytes, FAS-induced apoptosis requires amplification through proteolytic activation of the proapoptotic BCL-2 family member BID. Curiously, several components of the FAS signaling machinery have been implicated in nonapoptotic processes, including cellular activation, differentiation, and proliferation. This review describes current understanding of Fas-induced apoptosis signaling and proposes experimental strategies for future advances.
Figures


References
-
- Adachi M, Suematsu S, Kondo T, Ogasawara J, Tanaka T, Yoshida N, Nagata S. Targeted mutation in the Fas gene causes hyperplasia in peripheral lymphoid organs and liver. Nature Genetics. 1995;11:294–300. - PubMed
-
- Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003;3:745–756. - PubMed
-
- Aggarwal BB, Singh S, LaPushin R, Totpal K. Fas antigen signals proliferation of normal human diploid fibroblast and its mechanism is different from tumor necrosis factor receptor. FEBS Letters. 1995;364:5–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

