The ubiquitin-interacting motif protein, S5a, is ubiquitinated by all types of ubiquitin ligases by a mechanism different from typical substrate recognition
- PMID: 19240029
- PMCID: PMC2675991
- DOI: 10.1074/jbc.M900556200
The ubiquitin-interacting motif protein, S5a, is ubiquitinated by all types of ubiquitin ligases by a mechanism different from typical substrate recognition
Abstract
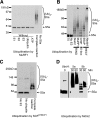
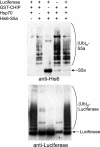
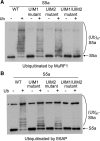
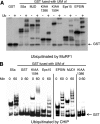
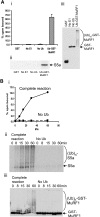
S5a/Rpn10 is a ubiquitin (Ub)-binding protein that is a subunit of the 26S proteasome but also exists free in the cytosol. It binds poly-Ub chains through its two Ub-interacting motifs (UIMs). We discovered that, unlike typical substrates of Ub ligases (E3s), S5a can be ubiquitinated by all E3s tested including multimeric and monomeric Ring finger E3s (MuRF1, Siah2, Parkin, APC, and SCF(betaTRCP1)), the U-box E3, CHIP, and HECT domain E3s (E6AP and Nedd4) when assayed with UbcH5 or related Ub-conjugating enzymes. However, the E2s, UbcH1 and UbcH13/Uev1a, which function by distinct mechanisms, do not support S5a ubiquitination. Thus, S5a can be used for assay of probably all E3s with UbcH5. Ubiquitination of S5a results from its binding to Ub chains on the E3 (after self-ubiquitination) or on the substrate, as a mutant lacking the UIM domain was not ubiquitinated. Furthermore, if the S5a UIM domains were fused to GST, the protein was rapidly ubiquitinated by MuRF1 and CHIP. In addition, polyubiquitination (but not monoubiquitination) of MuRF1 allowed S5a to bind to MuRF1 and accelerated S5a ubiquitination. This tendency of S5a to associate with the growing Ub chain can explain how S5a, unlike typical substrates, which are recognized by certain E3s through specific motifs, is ubiquitinated by all E3s tested and is rapidly degraded in vivo.
Figures


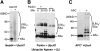
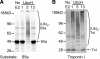
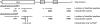
References
-
- Glickman, M. H., and Ciechanover, A. (2002) Physiol. Rev. 82 373–428 - PubMed
-
- Goldberg, A. L. (2003) Nature 426 895–899 - PubMed
-
- Pickart, C. M. (2001) Annu. Rev. Biochem. 70 503–533 - PubMed
-
- Varshavsky, A. (1997) Genes Cells 2 13–28 - PubMed
-
- Nash, P., Tang, X., Orlicky, S., Chen, Q., Gertler, F. B., Mendenhall, M. D., Sicheri, F., Pawson, T., and Tyers, M. (2001) Nature 414 514–521 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

