A novel role for SED1 (MFG-E8) in maintaining the integrity of the epididymal epithelium
- PMID: 19240116
- PMCID: PMC2714427
- DOI: 10.1242/jcs.041731
A novel role for SED1 (MFG-E8) in maintaining the integrity of the epididymal epithelium
Abstract
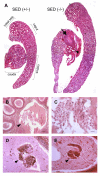
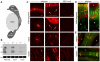
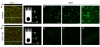
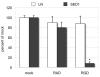
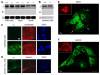
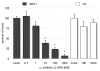
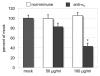
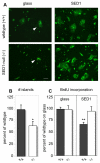
The epididymis is a highly convoluted tubule that connects the testis with the vas deferens, and in which mammalian sperm acquire the ability to fertilize eggs. The most proximal portion of the epididymis, or initial segment, secretes numerous factors that are critical for sperm maturation and storage. One such factor is SED1 (also known as MFG-E8) a bi-motif protein composed of two N-terminal EGF domains, the second of which contains an RGD motif, and two C-terminal discoidin domains (also known as F5/8 type C domains). Previous studies have reported that SED1 is secreted into the epididymal lumen, where it coats sperm and later facilitates sperm-egg binding. Herein, we report that SED1-null males also harbor unexpected epididymal pathologies, including detached epithelia and spermatic granulomas. We therefore examined whether SED1 has a tissue-intrinsic role in the epididymis, in addition to its role in sperm-egg adhesion. Improved fixation protocols revealed that SED1 is found in the basolateral domains of epididymal epithelial cells in vivo, and similarly, SED1 is secreted both apically and basally from polarized epididymal cells in vitro. The basolateral distribution of SED1 suggests that it may play a novel role in epididymal cell adhesion. Consistent with this, in vitro assays showed that SED1 supports epididymal cell adhesion via RGD binding to alphaV integrin receptors on epididymal epithelial cells. Finally, epididymal cells from SED1-null males showed reduced adhesion in vitro, a phenotype that can be rescued with exogenous SED1. These results suggest that SED1 facilitates epididymal cell adhesion, and that its loss leads to breakdown of the epididymal epithelium and consequent development of spermatic granulomas.
Figures


References
-
- Abe, K., Takano, H. and Ito, T. (1983). Ultrastructure of the mouse epididymal duct with special reference to the regional differences of the principal cells. Arch. Histol. Jpn. 46, 51-68. - PubMed
-
- Abou-Haèila, A. and Fain-Maurel, M. A. (1984). Regional differences of the proximal part of mouse epididymis: morphological and histochemical characterization. Anat. Rec. 209, 197-208. - PubMed
-
- Andersen, M. H., Berglund, L., Rasmussen, J. T. and Petersen, T. E. (1997). Bovine PAS-6/7 binds alpha v beta 5 integrins and anionic phospholipids through two domains. Biochemistry 36, 5441-5446. - PubMed
-
- Andersen, M. H., Graversen, H., Fedosov, S. N., Petersen, T. E. and Rasmussen, J. T. (2000). Functional analyses of two cellular binding domains of bovine lactadherin. Biochemistry 39, 6200-6206. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

