Flagellar membrane localization via association with lipid rafts
- PMID: 19240119
- PMCID: PMC2714428
- DOI: 10.1242/jcs.037721
Flagellar membrane localization via association with lipid rafts
Abstract
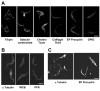
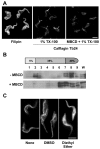
The eukaryotic flagellar membrane has a distinct composition from other domains of the plasmalemma. Our work shows that the specialized composition of the trypanosome flagellar membrane reflects increased concentrations of sterols and saturated fatty acids, correlating with direct observation of high liquid order by laurdan fluorescence microscopy. These findings indicate that the trypanosome flagellar membrane possesses high concentrations of lipid rafts: discrete regions of lateral heterogeneity in plasma membranes that serve to sequester and organize specialized protein complexes. Consistent with this, a dually acylated Ca(2+) sensor that is concentrated in the flagellum is found in detergent-resistant membranes and mislocalizes if the lipid rafts are disrupted. Detergent-extracted cells have discrete membrane patches localized on the surface of the flagellar axoneme, suggestive of intraflagellar transport particles. Together, these results provide biophysical and biochemical evidence to indicate that lipid rafts are enriched in the trypanosome flagellar membrane, providing a unique mechanism for flagellar protein localization and illustrating a novel means by which specialized cellular functions may be partitioned to discrete membrane domains.
Figures



Similar articles
-
Intraflagellar transport is required for the maintenance of the trypanosome flagellum composition but not its length.J Cell Sci. 2016 Aug 1;129(15):3026-41. doi: 10.1242/jcs.188227. Epub 2016 Jun 24. J Cell Sci. 2016. PMID: 27343245
-
The flagella connector of Trypanosoma brucei: an unusual mobile transmembrane junction.J Cell Sci. 2004 Apr 1;117(Pt 9):1641-51. doi: 10.1242/jcs.00995. Epub 2004 Mar 9. J Cell Sci. 2004. PMID: 15075226
-
Calcium-dependent membrane association of a flagellar calcium sensor does not require calcium binding.Mol Biochem Parasitol. 2015 May;201(1):72-75. doi: 10.1016/j.molbiopara.2015.06.003. Epub 2015 Jun 19. Mol Biochem Parasitol. 2015. PMID: 26099941 Free PMC article.
-
The trypanosome flagellar pocket.Nat Rev Microbiol. 2009 Nov;7(11):775-86. doi: 10.1038/nrmicro2221. Epub 2009 Oct 6. Nat Rev Microbiol. 2009. PMID: 19806154 Review.
-
Lipid Rafts and Plant Gravisensitivity.Life (Basel). 2022 Nov 7;12(11):1809. doi: 10.3390/life12111809. Life (Basel). 2022. PMID: 36362962 Free PMC article. Review.
Cited by
-
The plasma membrane of bloodstream-form African trypanosomes confers susceptibility and specificity to killing by hydrophobic peptides.J Biol Chem. 2010 Sep 10;285(37):28659-66. doi: 10.1074/jbc.M110.151886. Epub 2010 Jul 8. J Biol Chem. 2010. PMID: 20615879 Free PMC article.
-
Pharmacological cholesterol depletion disturbs ciliogenesis and ciliary function in developing zebrafish.Commun Biol. 2019 Jan 29;2:31. doi: 10.1038/s42003-018-0272-7. eCollection 2019. Commun Biol. 2019. PMID: 30729178 Free PMC article.
-
The Lipid Raft Proteome of African Trypanosomes Contains Many Flagellar Proteins.Pathogens. 2017 Aug 24;6(3):39. doi: 10.3390/pathogens6030039. Pathogens. 2017. PMID: 28837104 Free PMC article.
-
CiliaCarta: An integrated and validated compendium of ciliary genes.PLoS One. 2019 May 16;14(5):e0216705. doi: 10.1371/journal.pone.0216705. eCollection 2019. PLoS One. 2019. PMID: 31095607 Free PMC article.
-
Lipid synthesis in protozoan parasites: a comparison between kinetoplastids and apicomplexans.Prog Lipid Res. 2013 Oct;52(4):488-512. doi: 10.1016/j.plipres.2013.06.003. Epub 2013 Jul 1. Prog Lipid Res. 2013. PMID: 23827884 Free PMC article. Review.
References
-
- Anderson, R. G and Jacobson, K. (2002). A role for lipid shells in targeting proteins to caveolae, rafts, and other lipid domains. Science 296, 1821-1825. - PubMed
-
- Ames, J. B., Tanaka, T., Stryer, L. and Ikura, M. (1996). Portrait of a myristoyl switch protein. Curr. Opin. Struct. Biol. 6, 432-438. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

