The human CDK8 subcomplex is a molecular switch that controls Mediator coactivator function
- PMID: 19240132
- PMCID: PMC2648653
- DOI: 10.1101/gad.1767009
The human CDK8 subcomplex is a molecular switch that controls Mediator coactivator function
Abstract
The human CDK8 subcomplex (CDK8, cyclin C, Med12, and Med13) negatively regulates transcription in ways not completely defined; past studies suggested CDK8 kinase activity was required for its repressive function. Using a reconstituted transcription system together with recombinant or endogenous CDK8 subcomplexes, we demonstrate that, in fact, Med12 and Med13 are critical for subcomplex-dependent repression, whereas CDK8 kinase activity is not. A hallmark of activated transcription is efficient reinitiation from promoter-bound scaffold complexes that recruit a series of pol II enzymes to the gene. Notably, the CDK8 submodule strongly represses even reinitiation events, suggesting a means to fine tune transcript levels. Structural and biochemical studies confirm the CDK8 submodule binds the Mediator leg/tail domain via the Med13 subunit, and this submodule-Mediator association precludes pol II recruitment. Collectively, these results reveal the CDK8 subcomplex functions as a simple switch that controls the Mediator-pol II interaction to help regulate transcription initiation and reinitiation events. As Mediator is generally required for expression of protein-coding genes, this may reflect a common mechanism by which activated transcription is shut down in human cells.
Figures



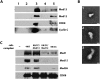
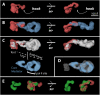

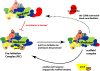
References
-
- Akoulitchev S., Chuikov S., Reinberg D. TFIIH is negatively regulated by cdk8-containing mediator complexes. Nature. 2000;407:102–106. - PubMed
-
- An W., Kim J., Roeder R.G. Ordered cooperative functions of PRMT1, p300, and CARM1 in transcriptional activation by p53. Cell. 2004;117:735–748. - PubMed
-
- Andrau J., van de Pasch L., Lijnzaad P., Bijma T., Koerkamp M.G., van de Peppel J., Werner M., Holstege F.C.P. Genome-wide location of the coactivator Mediator: Binding without activation and transient cdk8 interaction on DNA. Mol. Cell. 2006;22:179–192. - PubMed
-
- Berger S.L. The complex language of chromatin regulation during transcription. Nature. 2007;447:407–412. - PubMed
-
- Borggrefe T., Davis R., Erdjument-Bromage H., Tempst P., Kornberg R.D. A complex of the Srb8, -9, -10, and -11 transcriptional regulatory proteins from yeast. J. Biol. Chem. 2002;277:44202–44207. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
