Regulation of SR protein phosphorylation and alternative splicing by modulating kinetic interactions of SRPK1 with molecular chaperones
- PMID: 19240134
- PMCID: PMC2648651
- DOI: 10.1101/gad.1752109
Regulation of SR protein phosphorylation and alternative splicing by modulating kinetic interactions of SRPK1 with molecular chaperones
Abstract
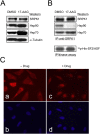
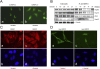
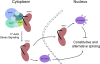
Phosphorylation is essential for the SR family of splicing factors/regulators to function in constitutive and regulated pre-mRNA splicing; yet both hypo- and hyperphosphorylation of SR proteins are known to inhibit splicing, indicating that SR protein phosphorylation must be tightly regulated in the cell. However, little is known how SR protein phosphorylation might be regulated during development or in response to specific signaling events. Here, we report that SRPK1, a ubiquitously expressed SR protein-specific kinase, directly binds to the cochaperones Hsp40/DNAjc8 and Aha1, which mediate dynamic interactions of the kinase with the major molecular chaperones Hsp70 and Hsp90 in mammalian cells. Inhibition of the Hsp90 ATPase activity induces dissociation of SRPK1 from the chaperone complexes, which can also be triggered by a stress signal (osmotic shock), resulting in translocation of the kinase from the cytoplasm to the nucleus, differential phosphorylation of SR proteins, and alteration of splice site selection. These findings connect the SRPK to the molecular chaperone system that has been implicated in numerous signal transduction pathways and provide mechanistic insights into complex regulation of SR protein phosphorylation and alternative splicing in response to developmental cues and cellular signaling.
Figures




References
-
- Cardinali B., Cohen P.T., Lamond A.I. Protein phosphatase 1 can modulate alternative 5′ splice site selection in a HeLa splicing extract. FEBS Lett. 1994;352:276–280. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
