Modulation of effector caspase cleavage determines response of breast and lung tumor cell lines to chemotherapy
- PMID: 19241192
- PMCID: PMC2914624
- DOI: 10.1080/07357900802438585
Modulation of effector caspase cleavage determines response of breast and lung tumor cell lines to chemotherapy
Abstract
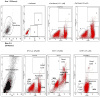
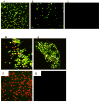
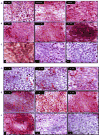
In spite of compelling evidence-implicating caspases in drug-induced apoptosis, how tumors modulate caspase expression and activity to overcome the cytotoxicity of anticancer agents is not fully understood. To address this issue, we investigated the role of caspases-3 and caspase-7 in determining the response of breast and lung tumor cell lines to chemotherapy. We found that an early and late apoptotic response correlated with weak and strong cellular caspase-activation, respectively. The results highlight an underappreciated relationship of temporal apoptotic response with caspase-activation and drug resistance. Moreover, the extent of tumor growth restoration after drug withdrawal was dependent on the degree of endogenous blockage of caspase-3 and caspase-7 cleavages. This points to an unrecognized role of caspase modulation in tumor recurrence and suggests that targeting caspase cleavage is a rational approach to increasing potency of cancer drugs.
Figures





Similar articles
-
Nuclear caspase-3 and caspase-7 activation, and poly(ADP-ribose) polymerase cleavage are early events in camptothecin-induced apoptosis.Apoptosis. 2006 Jan;11(1):131-9. doi: 10.1007/s10495-005-3276-y. Apoptosis. 2006. PMID: 16374543
-
Induction of apoptosis in human lung carcinoma cells by the water extract of Panax notoginseng is associated with the activation of caspase-3 through downregulation of Akt.Int J Oncol. 2009 Jul;35(1):121-7. doi: 10.3892/ijo_00000320. Int J Oncol. 2009. PMID: 19513559
-
Mitochondrial dysfunction is an essential step for killing of non-small cell lung carcinomas resistant to conventional treatment.Oncogene. 2002 Jan 3;21(1):65-77. doi: 10.1038/sj.onc.1205018. Oncogene. 2002. PMID: 11791177
-
Dietary flavonoid fisetin targets caspase-3-deficient human breast cancer MCF-7 cells by induction of caspase-7-associated apoptosis and inhibition of autophagy.Int J Oncol. 2012 Feb;40(2):469-78. doi: 10.3892/ijo.2011.1203. Epub 2011 Sep 15. Int J Oncol. 2012. PMID: 21922137
-
Lipid-soluble ginseng extract induces apoptosis and G0/G1 cell cycle arrest in NCI-H460 human lung cancer cells.Plant Foods Hum Nutr. 2011 Jun;66(2):101-6. doi: 10.1007/s11130-011-0232-6. Plant Foods Hum Nutr. 2011. PMID: 21611769
Cited by
-
A traditional Chinese medicine compound (Jian Er) for presbycusis in a mouse model: Reduction of apoptosis and protection of cochlear sensorineural cells and hearing.Int J Herb Med. 2018 Nov-Dec;6(6):127-135. Int J Herb Med. 2018. PMID: 31890893 Free PMC article.
-
Electroacupuncture Promoting Axonal Regeneration in Spinal Cord Injury Rats via Suppression of Nogo/NgR and Rho/ROCK Signaling Pathway.Neuropsychiatr Dis Treat. 2019 Dec 13;15:3429-3442. doi: 10.2147/NDT.S216874. eCollection 2019. Neuropsychiatr Dis Treat. 2019. PMID: 31997879 Free PMC article.
-
A Novel 5-Chloro-N-phenyl-1H-indole-2-carboxamide Derivative as Brain-Type Glycogen Phosphorylase Inhibitor: Validation of Target PYGB.Molecules. 2023 Feb 10;28(4):1697. doi: 10.3390/molecules28041697. Molecules. 2023. PMID: 36838691 Free PMC article.
-
Hepatoprotective Activity of Vitamin E and Metallothionein in Cadmium-Induced Liver Injury in Ctenopharyngodon idellus.Oxid Med Cell Longev. 2018 Apr 11;2018:9506543. doi: 10.1155/2018/9506543. eCollection 2018. Oxid Med Cell Longev. 2018. PMID: 29849926 Free PMC article.
-
Immunohistochemical study of NF-κB p65, c-IAP2 and caspase-3 expression in cervical cancer.Oncol Lett. 2012 Apr 1;3(4):839-844. doi: 10.3892/ol.2012.564. Epub 2012 Jan 12. Oncol Lett. 2012. PMID: 22741003 Free PMC article.
References
-
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(2):74–108. - PubMed
-
- Yoh K, Ishii G, Yokose T, et al. Breast cancer resistance protein impacts clinical outcome in platinum-based chemotherapy for advanced non-small cell lung cancer. Clin Cancer Res. 2004;10(5):1691–1697. - PubMed
-
- Gee MS, Upadhyay R, Bergquist H, et al. Multiparameter noninvasive assessment of treatment susceptibility, drug target inhibition and tumor response guides cancer treatment. Int J Cancer. 2007;121(11):2492–2500. - PubMed
-
- Janssen K, Pohlmann S, Janicke RU, Schulze-Osthoff K, Fischer U. Apaf-1 and caspase-9 deficiency prevents apoptosis in a Bax-controlled pathway and promotes clonogenic survival during paclitaxel treatment. Blood. 2007;110(10):3662–3672. - PubMed
-
- Wieder T, Essmann F, Prokop A, et al. Activation of caspase-8 in drug-induced apoptosis of B-lymphoid cells is independent of CD95/Fas receptor-ligand interaction and occurs downstream of caspase-3. Blood. 2001;97(5):1378–1387. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
