Probabilistic topography of human corpus callosum using cytoarchitectural parcellation and high angular resolution diffusion imaging tractography
- PMID: 19241418
- PMCID: PMC6871153
- DOI: 10.1002/hbm.20739
Probabilistic topography of human corpus callosum using cytoarchitectural parcellation and high angular resolution diffusion imaging tractography
Abstract
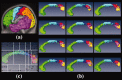
The function of the corpus callosum (CC) is to distribute perceptual, motor, cognitive, learned, and voluntary information between the two hemispheres of the brain. Accurate parcellation of the CC according to fiber composition and fiber connection is of upmost important. In this work, population-based probabilistic connection topographies of the CC, in the standard Montreal Neurological Institute (MNI) space, are estimated by incorporating anatomical cytoarchitectural parcellation with high angular resolution diffusion imaging (HARDI) tractography. First, callosal fibers are extracted using multiple fiber assignment by continuous tracking algorithm based on q-ball imaging (QBI), on 12 healthy and young subjects. Then, the fiber tracts are aligned in the standard MNI coordinate system based on a tract-based transformation scheme. Next, twenty-eight Brodmann's areas on the surface of cortical cortex are registered to the MNI space to parcellate the aligned callosal fibers. Finally, the population-based topological subdivisions of the midsagittal CC to each cortical target are then mapped. And the resulting subdivisions of the CC that connect to the frontal and somatosensory associated cortex are also showed. To our knowledge, it is the first topographic subdivisions of the CC done using HARDI tractography and cytoarchitectonic information. In conclusion, this sophisticated topography of the CC may serve as a landmark to further understand the correlations between the CC, brain intercommunication, and functional cytoarchitectures.
Figures
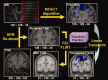
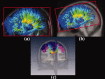
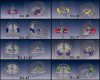

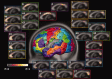

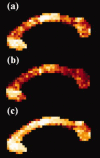
References
-
- Abe O,Masutani Y,Aoki S,Yamasue H,Yamada H,Kasai K,Mori H,Hayashi N,Masumoto T,Ohtomo K ( 2004): Topography of the human corpus callosum using diffusion tensor tractography. J Comput Assist Tomogr 28: 533–539. - PubMed
-
- Alexander DC ( 2005): Multiple‐fiber reconstruction algorithms for diffusion MRI. Ann N Y Acad Sci 1064: 113–133. - PubMed
-
- Alexander DC,Pierpaoli C,Basser PJ,Gee JC ( 2001): Spatial transformations of diffusion tensor magnetic resonance images. IEEE Trans Med Imaging 20: 1131–1139. - PubMed
-
- Alexander AL,Lee JE,Lazar M,Boudos R,DuBray MB,Oakes TR,Miller JN,Lu J,Jeong EK,McMahon WM,Bigler ED,Lainhart JE ( 2007): Diffusion tensor imaging of the corpus callosum in Autism. Neuroimage 34: 61–73. - PubMed
-
- Amunts K,Malikovic A,Mohlberg H,Schormann T,Zilles K ( 2000): Brodmann's areas 17 and 18 brought into stereotaxic space—Where and how variable? Neuroimage 11: 66–84. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

