Learning therapeutic lessons from metastasis suppressor proteins
- PMID: 19242414
- PMCID: PMC2881216
- DOI: 10.1038/nrc2594
Learning therapeutic lessons from metastasis suppressor proteins
Abstract
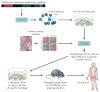
Metastasis suppressor proteins regulate multiple steps in the metastatic cascade, including cancer cell invasion, survival in the vascular and lymphatic circulation, and colonization of distant organ sites. Understanding the biology of metastasis suppressors provides valuable mechanistic insights that may translate to therapeutic opportunities. Several reports have explored novel strategies for restoring metastasis suppressor function, including gene transfer, induction of previously suppressed gene expression and exogenous administration of gene product. Pathways activated downstream of metastasis suppressor loss can also be targeted. Although none of these strategies are yet in routine clinical use, several are being tested preclinically and in clinical trials.
Figures




References
-
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. - PubMed
-
- Gupta GP, Massague J. Cancer metastasis: building a framework. Cell. 2006;127:679–695. - PubMed
-
- Fidler IJ, Poste G. The “seed and soil” hypothesis revisited. Lancet Oncol. 2008;9:808. - PubMed
-
- Steeg PS. Metastasis suppressors alter the signal transduction of cancer cells. Nature Rev Cancer. 2003;3:55–63. - PubMed
-
- Stafford LJ, Vaidya KS, Welch DR. Metastasis suppressors genes in cancer. Int J Biochem Cell Biol. 2008;40:874–891. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

