A human trial of HSV-mediated gene transfer for the treatment of chronic pain
- PMID: 19242524
- PMCID: PMC2683467
- DOI: 10.1038/gt.2009.17
A human trial of HSV-mediated gene transfer for the treatment of chronic pain
Abstract
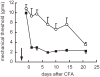
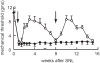
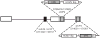
Gene transfer to the dorsal root ganglion using replication defective herpes simplex virus (HSV)-based vectors reduces pain-related behaviors in rodent models having inflammatory pain, neuropathic pain and pain caused by cancer in bone. HSV vectors engineered to produce inhibitory neurotransmitters, including the delta opioid agonist peptide enkephalin, the mu opioid agonist peptide endomorphin-2 and glutamic acid decarboxylase (GAD), to effect the release of gamma amino butyric acid (GABA) act to inhibit nociceptive neurotransmission at the first synapse between primary nociceptive and second-order neuron in the dorsal horn of the spinal cord. HSV vectors engineered to release anti-inflammatory peptides, including interleukin (IL)-4, IL-10 and the p55 soluble tumor necrosis factor alpha (TNFalpha) receptor reduce neuroimmune activation in the spinal dorsal horn. The path leading from preclinical animal studies to the ongoing phase 1 human trial of the enkephalin-producing vector in patients with pain from cancer, and plans for an efficacy trial with an opioid-producing vector in inflammatory pain and an efficacy trial with a GAD-producing vector in diabetic neuropathic pain are outlined.
Conflict of interest statement
Dr. Mata has no conflict of interest to declare.
Figures
Similar articles
-
Gene Transfer of Glutamic Acid Decarboxylase 67 by Herpes Simplex Virus Vectors Suppresses Neuropathic Pain Induced by Human Immunodeficiency Virus gp120 Combined with ddC in Rats.Anesth Analg. 2015 Jun;120(6):1394-404. doi: 10.1213/ANE.0000000000000729. Anesth Analg. 2015. PMID: 25851180
-
Vector-mediated gene transfer to express inhibitory neurotransmitters in dorsal root ganglion reduces pain in a rodent model of lumbar radiculopathy.Spine (Phila Pa 1976). 2006 Jun 15;31(14):1555-8. doi: 10.1097/01.brs.0000222060.88919.58. Spine (Phila Pa 1976). 2006. PMID: 16778687
-
Peripherally delivered glutamic acid decarboxylase gene therapy for spinal cord injury pain.Mol Ther. 2004 Jul;10(1):57-66. doi: 10.1016/j.ymthe.2004.04.017. Mol Ther. 2004. PMID: 15233942
-
Exploiting the neurotherapeutic potential of peptides: targeted delivery using HSV vectors.Expert Opin Biol Ther. 2003 Dec;3(8):1233-9. doi: 10.1517/14712598.3.8.1233. Expert Opin Biol Ther. 2003. PMID: 14640949 Review.
-
HSV gene transfer in the treatment of chronic pain.Sheng Li Xue Bao. 2008 Oct 25;60(5):610-6. Sheng Li Xue Bao. 2008. PMID: 18958369 Free PMC article. Review.
Cited by
-
Therapeutic application of the CRISPR system: current issues and new prospects.Hum Genet. 2019 Jun;138(6):563-590. doi: 10.1007/s00439-019-02028-2. Epub 2019 May 21. Hum Genet. 2019. PMID: 31115652 Review.
-
Managing the placebo effect: enhancing the signal-to-noise ratio.Curr Pain Headache Rep. 2011 Feb;15(1):35-8. doi: 10.1007/s11916-010-0162-2. Curr Pain Headache Rep. 2011. PMID: 21153719 Review.
-
A clinical trial of gene therapy for chronic pain.Pain Med. 2009 Oct;10(7):1325-30. doi: 10.1111/j.1526-4637.2009.00720.x. Pain Med. 2009. PMID: 19818042 Free PMC article. Review.
-
Vector-mediated expression of erythropoietin improves functional outcome after cervical spinal cord contusion injury.Gene Ther. 2012 Sep;19(9):907-14. doi: 10.1038/gt.2011.166. Epub 2011 Nov 3. Gene Ther. 2012. PMID: 22052241 Free PMC article.
-
Gene delivery with viral vectors for cerebrovascular diseases.Front Biosci (Elite Ed). 2013 Jan 1;5(1):188-203. doi: 10.2741/e607. Front Biosci (Elite Ed). 2013. PMID: 23276981 Free PMC article. Review.
References
-
- Wolfe D, Goins WF, Yamada M, Moriuchi S, Krisky DM, Oligino TJ, et al. Engineering herpes simplex virus vectors for CNS applications. Exp Neurol. 1999;159:34–46. - PubMed
-
- Glorioso JC, Fink DJ. Herpes vector-mediated gene transfer in treatment of diseases of the nervous system. Annu Rev Microbiol. 2004;58:253–271. - PubMed
-
- Jasmin L, Rabkin SD, Granato A, Boudah A, Ohara PT. Analgesia and hyperalgesia from GABA-mediated modulation of the cerebral cortex. Nature. 2003;424:316–320. - PubMed
-
- Kang W, Wilson MA, Bender MA, Glorioso JC, Wilson SP. Herpes virus-mediated preproenkephalin gene transfer to the amygdala is antinociceptive. Brain Res. 1998;792:133–135. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

