Antigen-binding properties of monoclonal antibodies reactive with EBNA1 and use in immunoaffinity chromatography
- PMID: 19242546
- PMCID: PMC2644765
- DOI: 10.1371/journal.pone.0004614
Antigen-binding properties of monoclonal antibodies reactive with EBNA1 and use in immunoaffinity chromatography
Abstract
Epstein-Barr virus (EBV) nuclear antigen 1 (EBNA1) was overexpressed and purified from Escherichia coli. Mouse monoclonal antibodies (mAbs) were prepared that react with EBNA1. Eleven high affinity mAbs were recovered. Nine mAbs are isotype IgG (all subisotype IgG(1)) and two mAbs are isotype IgM. All mAbs react strongly with EBNA1 in an ELISA assay while only one mAb (designated 1EB6) fails to react in a Western blot assay. The epitopes for these mAbs were mapped to seven different regions, providing good coverage of the entire EBNA1 protein. The mAbs had differing affinity for an EBNA1/DNA complex with four mAbs able to supershift the complex completely. All mAbs can immunoprecipitate EBNA1 from E. coli overexpressing EBNA1. A modified ELISA assay, termed ELISA-elution assay, was used to screen for mAbs that release EBNA1 in the presence of a low molecular weight polyhydroxylated compound (polyol) and a nonchaotropic salt. MAbs with this property, termed polyol-responsive (PR)-mAbs, allow gentle elution of labile proteins and protein complexes. Four mAbs are polyol-responsive with two showing usefulness in gentle immunoaffinity chromatography. Purification with these PR-mAbs may be useful in purifying EBNA1 complexes and elucidating EBNA1-associated proteins. This panel of anti-EBNA1 mAbs will advance the study of EBV by providing new tools to detect and purify EBNA1.
Conflict of interest statement
Figures

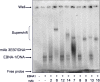
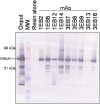
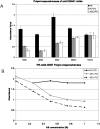

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

