Signaling logic of activity-triggered dendritic protein synthesis: an mTOR gate but not a feedback switch
- PMID: 19242559
- PMCID: PMC2647780
- DOI: 10.1371/journal.pcbi.1000287
Signaling logic of activity-triggered dendritic protein synthesis: an mTOR gate but not a feedback switch
Abstract
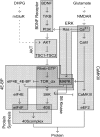
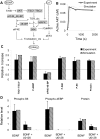
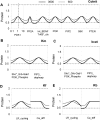
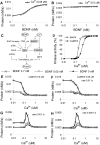
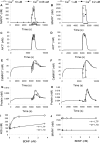
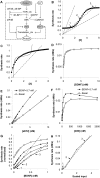
Changes in synaptic efficacy are believed to form the cellular basis for memory. Protein synthesis in dendrites is needed to consolidate long-term synaptic changes. Many signals converge to regulate dendritic protein synthesis, including synaptic and cellular activity, and growth factors. The coordination of these multiple inputs is especially intriguing because the synthetic and control pathways themselves are among the synthesized proteins. We have modeled this system to study its molecular logic and to understand how runaway feedback is avoided. We show that growth factors such as brain-derived neurotrophic factor (BDNF) gate activity-triggered protein synthesis via mammalian target of rapamycin (mTOR). We also show that bistability is unlikely to arise from the major protein synthesis pathways in our model, even though these include several positive feedback loops. We propose that these gating and stability properties may serve to suppress runaway activation of the pathway, while preserving the key role of responsiveness to multiple sources of input.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Sutton MA, Schuman EM. Dendritic protein synthesis, synaptic plasticity, and memory. Cell. 2006;127:49–58. - PubMed
-
- Bramham CR, Wells DG. Dendritic mRNA: transport, translation and function. Nat Rev Neurosci. 2007;8:776–789. - PubMed
-
- Klann E, Antion MD, Banko JL, Hou L. Synaptic plasticity and translation initiation. Learn Mem. 2004;11:365–372. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

