HCV induces oxidative and ER stress, and sensitizes infected cells to apoptosis in SCID/Alb-uPA mice
- PMID: 19242562
- PMCID: PMC2647842
- DOI: 10.1371/journal.ppat.1000291
HCV induces oxidative and ER stress, and sensitizes infected cells to apoptosis in SCID/Alb-uPA mice
Abstract
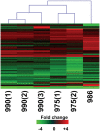
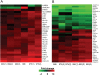
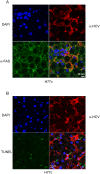
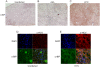
Hepatitis C virus (HCV) is a blood-borne pathogen and a major cause of liver disease worldwide. Gene expression profiling was used to characterize the transcriptional response to HCV H77c infection. Evidence is presented for activation of innate antiviral signaling pathways as well as induction of lipid metabolism genes, which may contribute to oxidative stress. We also found that infection of chimeric SCID/Alb-uPA mice by HCV led to signs of hepatocyte damage and apoptosis, which in patients plays a role in activation of stellate cells, recruitment of macrophages, and the subsequent development of fibrosis. Infection of chimeric mice with HCV H77c also led an inflammatory response characterized by infiltration of monocytes and macrophages. There was increased apoptosis in HCV-infected human hepatocytes in H77c-infected mice but not in mice inoculated with a replication incompetent H77c mutant. Moreover, TUNEL reactivity was restricted to HCV-infected hepatocytes, but an increase in FAS expression was not. To gain insight into the factors contributing specific apoptosis of HCV infected cells, immunohistological and confocal microscopy using antibodies for key apoptotic mediators was done. We found that the ER chaperone BiP/GRP78 was increased in HCV-infected cells as was activated BAX, but the activator of ER stress-mediated apoptosis CHOP was not. We found that overall levels of NF-kappaB and BCL-xL were increased by infection; however, within an infected liver, comparison of infected cells to uninfected cells indicated both NF-kappaB and BCL-xL were decreased in HCV-infected cells. We conclude that HCV contributes to hepatocyte damage and apoptosis by inducing stress and pro-apoptotic BAX while preventing the induction of anti-apoptotic NF-kappaB and BCL-xL, thus sensitizing hepatocytes to apoptosis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



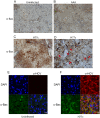

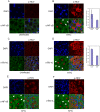
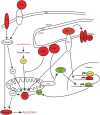
Similar articles
-
The Molecular Chaperone GRP78 Contributes to Toll-like Receptor 3-mediated Innate Immune Response to Hepatitis C Virus in Hepatocytes.J Biol Chem. 2016 Jun 3;291(23):12294-309. doi: 10.1074/jbc.M115.711598. Epub 2016 Apr 20. J Biol Chem. 2016. PMID: 27129228 Free PMC article.
-
Hepatitis B and C virus-induced hepatitis: Apoptosis, autophagy, and unfolded protein response.World J Gastroenterol. 2015 Dec 21;21(47):13225-39. doi: 10.3748/wjg.v21.i47.13225. World J Gastroenterol. 2015. PMID: 26715805 Free PMC article.
-
Hepatitis C virus nonstructural protein 5A inhibits thapsigargin-induced apoptosis.PLoS One. 2014 Nov 19;9(11):e113499. doi: 10.1371/journal.pone.0113499. eCollection 2014. PLoS One. 2014. PMID: 25409163 Free PMC article.
-
Hepatitis C Virus Infection Induces Autophagy as a Prosurvival Mechanism to Alleviate Hepatic ER-Stress Response.Viruses. 2016 May 23;8(5):150. doi: 10.3390/v8050150. Viruses. 2016. PMID: 27223299 Free PMC article. Review.
-
Endoplasmic reticulum (ER) stress: hepatitis C virus induces an ER-nucleus signal transduction pathway and activates NF-kappaB and STAT-3.Biochem Pharmacol. 2002 Nov 15;64(10):1425-30. doi: 10.1016/s0006-2952(02)01300-x. Biochem Pharmacol. 2002. PMID: 12417255 Review.
Cited by
-
Integrated stress response in hepatitis C promotes Nrf2-related chaperone-mediated autophagy: A novel mechanism for host-microbe survival and HCC development in liver cirrhosis.Semin Cell Dev Biol. 2020 May;101:20-35. doi: 10.1016/j.semcdb.2019.07.015. Epub 2019 Aug 8. Semin Cell Dev Biol. 2020. PMID: 31386899 Free PMC article. Review.
-
Humanized Mouse Models for the Study of Hepatitis C and Host Interactions.Cells. 2019 Jun 17;8(6):604. doi: 10.3390/cells8060604. Cells. 2019. PMID: 31213010 Free PMC article. Review.
-
An insight into the diagnosis and pathogenesis of hepatitis C virus infection.World J Gastroenterol. 2013 Nov 28;19(44):7896-909. doi: 10.3748/wjg.v19.i44.7896. World J Gastroenterol. 2013. PMID: 24307784 Free PMC article. Review.
-
Different responses of two highly permissive cell lines upon HCV infection.Virol Sin. 2013 Aug;28(4):202-8. doi: 10.1007/s12250-013-3342-5. Epub 2013 Jun 27. Virol Sin. 2013. PMID: 23818110 Free PMC article.
-
Focal distribution of hepatitis C virus RNA in infected livers.PLoS One. 2009 Aug 18;4(8):e6661. doi: 10.1371/journal.pone.0006661. PLoS One. 2009. PMID: 19688046 Free PMC article.
References
-
- Alter MJ, Margolis HS, Krawczynski K, Judson FN, Mares A, et al. The natural history of community-acquired hepatitis C in the United States. The Sentinel Counties Chronic non-A, non-B Hepatitis Study Team. N Engl J Med. 1992;327:1899–1905. - PubMed
-
- Roberts MS, Angus DC, Bryce CL, Valenta Z, Weissfeld L. Survival after liver transplantation in the United States: a disease-specific analysis of the UNOS database. Liver Transpl. 2004;10:886–897. - PubMed
-
- Smith MW, Yue ZN, Korth MJ, Do HA, Boix L, et al. Hepatitis C virus and liver disease: global transcriptional profiling and identification of potential markers. Hepatology. 2003;38:1458–1467. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

