Protein quality control as a strategy for cellular regulation: lessons from ubiquitin-mediated regulation of the sterol pathway
- PMID: 19243134
- PMCID: PMC8530524
- DOI: 10.1021/cr800544v
Protein quality control as a strategy for cellular regulation: lessons from ubiquitin-mediated regulation of the sterol pathway
Figures
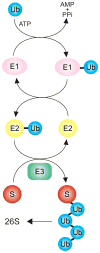
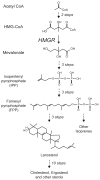


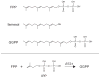


References
-
- Ciechanover A, Iwai K. IUBMB Life. 2004;56:193. - PubMed
-
- Richly H, Rape M, Braun S, Rumpf S, Hoege C, Jentsch S. Cell. 2005;120:73. - PubMed
-
- Elsasser S, Chandler-Militello D, Muller B, Hanna J, Finley D. J Biol Chem. 2004;279:26817. - PubMed
-
- Fang S, Jensen JP, Ludwig RL, Vousden KH, Weissman AM. J Biol Chem. 2000;275:8945. - PubMed
-
- Pines J. Trends Cell Biol. 2006;16:55. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

