Structural basis for inhibition of mammalian adenylyl cyclase by calcium
- PMID: 19243146
- PMCID: PMC2680196
- DOI: 10.1021/bi802122k
Structural basis for inhibition of mammalian adenylyl cyclase by calcium
Abstract
Type V and VI mammalian adenylyl cyclases (AC5, AC6) are inhibited by Ca(2+) at both sub- and supramicromolar concentration. This inhibition may provide feedback in situations where cAMP promotes opening of Ca(2+) channels, allowing fine control of cardiac contraction and rhythmicity in cardiac tissue where AC5 and AC6 predominate. Ca(2+) inhibits the soluble AC core composed of the C1 domain of AC5 (VC1) and the C2 domain of AC2 (IIC2). As observed for holo-AC5, inhibition is biphasic, showing "high-affinity" (K(i) = approximately 0.4 microM) and "low-affinity" (K(i) = approximately 100 microM) modes of inhibition. At micromolar concentration, Ca(2+) inhibition is nonexclusive with respect to pyrophosphate (PP(i)), a noncompetitive inhibitor with respect to ATP, but at >100 microM Ca(2+), inhibition appears to be exclusive with respect to PP(i). The 3.0 A resolution structure of Galphas.GTPgammaS/forskolin-activated VC1:IIC2 crystals soaked in the presence of ATPalphaS and 8 microM free Ca(2+) contains a single, loosely coordinated metal ion. ATP soaked into VC1:IIC2 crystals in the presence of 1.5 mM Ca(2+) is not cyclized, and two calcium ions are observed in the 2.9 A resolution structure of the complex. In both of the latter complexes VC1:IIC2 adopts the "open", catalytically inactive conformation characteristic of the apoenzyme, in contrast to the "closed", active conformation seen in the presence of ATP analogues and Mg(2+) or Mn(2+). Structures of the pyrophosphate (PP(i)) complex with 10 mM Mg(2+) (2.8 A) or 2 mM Ca(2+) (2.7 A) also adopt the open conformation, indicating that the closed to open transition occurs after cAMP release. In the latter complexes, Ca(2+) and Mg(2+) bind only to the high-affinity "B" metal site associated with substrate/product stabilization. Ca(2+) thus stabilizes the inactive conformation in both ATP- and PP(i)-bound states.
Figures





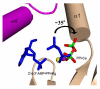
Similar articles
-
Structural basis for the inhibition of mammalian membrane adenylyl cyclase by 2 '(3')-O-(N-Methylanthraniloyl)-guanosine 5 '-triphosphate.J Biol Chem. 2005 Feb 25;280(8):7253-61. doi: 10.1074/jbc.M409076200. Epub 2004 Dec 9. J Biol Chem. 2005. PMID: 15591060
-
Molecular basis for P-site inhibition of adenylyl cyclase.Biochemistry. 2000 Nov 28;39(47):14464-71. doi: 10.1021/bi0015562. Biochemistry. 2000. PMID: 11087399
-
Inhibition by calcium of mammalian adenylyl cyclases.J Biol Chem. 1999 Dec 10;274(50):35539-45. doi: 10.1074/jbc.274.50.35539. J Biol Chem. 1999. PMID: 10585428
-
Mammalian Nucleotidyl Cyclases and Their Nucleotide Binding Sites.Handb Exp Pharmacol. 2017;238:49-66. doi: 10.1007/164_2015_34. Handb Exp Pharmacol. 2017. PMID: 27900607 Review.
-
International Union of Basic and Clinical Pharmacology. CI. Structures and Small Molecule Modulators of Mammalian Adenylyl Cyclases.Pharmacol Rev. 2017 Apr;69(2):93-139. doi: 10.1124/pr.116.013078. Pharmacol Rev. 2017. PMID: 28255005 Free PMC article. Review.
Cited by
-
Adenylyl cyclase 5 links changes in calcium homeostasis to cAMP-dependent cyst growth in polycystic liver disease.J Hepatol. 2017 Mar;66(3):571-580. doi: 10.1016/j.jhep.2016.10.032. Epub 2016 Nov 5. J Hepatol. 2017. PMID: 27826057 Free PMC article.
-
Impact of divalent metal ions on regulation of adenylyl cyclase isoforms by forskolin analogs.Biochem Pharmacol. 2011 Dec 1;82(11):1673-81. doi: 10.1016/j.bcp.2011.07.099. Epub 2011 Aug 6. Biochem Pharmacol. 2011. PMID: 21843517 Free PMC article.
-
Structure of the Cmr2 subunit of the CRISPR-Cas RNA silencing complex.Structure. 2012 Mar 7;20(3):545-53. doi: 10.1016/j.str.2012.01.018. Structure. 2012. PMID: 22405013 Free PMC article.
-
Adenylyl cyclase isoforms 5 and 6 in the cardiovascular system: complex regulation and divergent roles.Front Pharmacol. 2024 Apr 3;15:1370506. doi: 10.3389/fphar.2024.1370506. eCollection 2024. Front Pharmacol. 2024. PMID: 38633617 Free PMC article. Review.
-
Cardiac cAMP: production, hydrolysis, modulation and detection.Front Pharmacol. 2015 Oct 1;6:203. doi: 10.3389/fphar.2015.00203. eCollection 2015. Front Pharmacol. 2015. PMID: 26483685 Free PMC article. Review.
References
-
- Sunahara RK, Dessauer CW, Gilman AG. Complexity and diversity of mammalian adenylyl cyclases. Annu Rev Pharmacol Toxicol. 1996;36:461–480. - PubMed
-
- Garbers DL, Johnson RA. Metal and metal-ATP interactions with brain and cardiac adenylate cyclases. J Biol Chem. 1975;250:8449–8456. - PubMed
-
- Zimmermann G, Zhou D, Taussig R. Mutations uncover a role for two magnesium ions in the catalytic mechanism of adenylyl cyclase. J Biol Chem. 1998;273:19650–19655. - PubMed
-
- Tesmer JJG, Sunahara RK, Johnson RA, Gilman AG, Sprang SR. Two metal ion catalysis in adenylyl cyclase. Science. 1999;285:756–760. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

