Two distinct signalling cascades target the NF-kappaB regulatory factor c-IAP1 for degradation
- PMID: 19243308
- PMCID: PMC2677214
- DOI: 10.1042/BJ20082140
Two distinct signalling cascades target the NF-kappaB regulatory factor c-IAP1 for degradation
Abstract
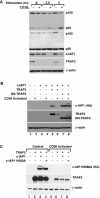
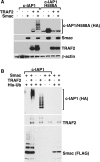
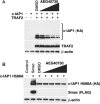
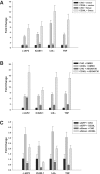
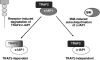
c-IAP1 (cellular inhibitor of apoptosis 1) has recently emerged as a negative regulator of the non-canonical NF-kappaB (nuclear factor kappaB) signalling cascade. Whereas synthetic IAP inhibitors have been shown to trigger the autoubiquitination and degradation of c-IAP1, less is known about the physiological mechanisms by which c-IAP1 stability is regulated. In the present paper, we describe two distinct cellular processes that lead to the targeted loss of c-IAP1. Recruitment of a TRAF2 (tumour necrosis factor receptor-associated factor 2)-c-IAP1 complex to the cytoplasmic domain of the Hodgkin's/anaplastic large-cell lymphoma-associated receptor, CD30, leads to the targeting and degradation of the TRAF2-c-IAP1 heterodimer through a mechanism requiring the RING (really interesting new gene) domain of TRAF2, but not c-IAP1. In contrast, the induced autoubiquitination of c-IAP1 by IAP antagonists causes the selective loss of c-IAP1, but not TRAF2, thereby releasing TRAF2. Thus c-IAP1 can be targeted for degradation by two distinct processes, revealing the critical importance of this molecule as a regulator of numerous intracellular signalling cascades.
Figures


References
-
- Stein H., Foss H. D., Dürkop H., Marafioti T., Delsol G., Pulford K., Pileri S., Falini B. CD30+ anaplastic large cell lymphoma: a review of its histopathologic, genetic, and clinical features. Blood. 2000;96:3681–3695. - PubMed
-
- Younes A., Carbone A. CD30/CD30 ligand and CD40/CD40 ligand in malignant lymphoid disorders. Int. J. Biol. Markers. 1999;14:135–143. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

