A2B5 cells from human glioblastoma have cancer stem cell properties
- PMID: 19243384
- PMCID: PMC8094826
- DOI: 10.1111/j.1750-3639.2009.00269.x
A2B5 cells from human glioblastoma have cancer stem cell properties
Abstract
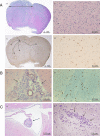
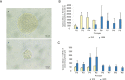
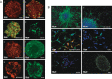
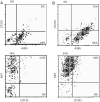
Glioblastomas, like other cancers, harbor small cell populations with the capability of sustaining tumor formation. These cells are referred to as cancer stem cells. We isolated cells expressing the surface marker A2B5 from three human glioblastomas (GBM) and showed that after grafting into nude mice, they generated dense and highly infiltrative tumors. Then, we extensively studied A2B5(+) cells isolated from 11 human GBM. These cells display neurosphere-like, self-renewal, asymmetrical cell division properties and have multipotency capability. Stereotactic xenografts of dissociated A2B5(+)-derived secondary spheres revealed that as few as 1000 cells produced a tumor. Moreover, flow cytometry characterization of A2B5(+)-derived spheres revealed three distinct populations of cells: A2B5(+)/CD133(+), A2B5(+)/CD133(-) and A2B5(-)/CD133(-), with striking proportion differences among GBM. Both A2B5(+)/CD133(+) and A2B5(+)/CD133(-) cell fractions displayed a high proliferative index, the potential to generate spheres and produced tumors in nude mice. Finally, we generated two green fluorescent protein-cell lines that display--after serum induction--distinct proliferative and migratory properties, and differ in their CD133 level of expression. Taken together, our results suggest that transformed A2B5(+) cells are crucial for the initiation and maintenance of GBM, although CD133 expression is more involved in determining the tumor's behavior.
Figures
References
-
- Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB et al (2006) Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 444:756–760. - PubMed
-
- Beier D, Hau P, Proescholdt M, Lohmeier A, Wischhusen J, Oefner PJ et al (2007) CD133(+) and CD133(−) glioblastoma‐derived cancer stem cells show differential growth characteristics and molecular profiles. Cancer Res 67:4010–4015. - PubMed
-
- Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B et al (2007) A perivascular niche for brain tumor stem cells. Cancer Cell 11:69–82. - PubMed
-
- Colin C, Baeza N, Tong S, Bouvier C, Quilichini B, Durbec P, Figarella‐Branger D (2006) In vitro identification and functional characterization of glial precursor cells in human gliomas. Neuropathol Appl Neurobiol 32:189–202. - PubMed
-
- Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S et al (2004) Isolation and characterization of tumorigenic, stem‐like neural precursors from human glioblastomas. Cancer Res 64:7011–7021. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

