Randomised Phase I/II trial assessing the safety and efficacy of radiolabelled anti-carcinoembryonic antigen I(131) KAb201 antibodies given intra-arterially or intravenously in patients with unresectable pancreatic adenocarcinoma
- PMID: 19243606
- PMCID: PMC2656541
- DOI: 10.1186/1471-2407-9-66
Randomised Phase I/II trial assessing the safety and efficacy of radiolabelled anti-carcinoembryonic antigen I(131) KAb201 antibodies given intra-arterially or intravenously in patients with unresectable pancreatic adenocarcinoma
Abstract
Background: Advanced pancreatic cancer has a poor prognosis, and the current standard of care (gemcitabine based chemotherapy) provides a small survival advantage. However the drawback is the accompanying systemic toxicity, which targeted treatments may overcome. This study aimed to evaluate the safety and tolerability of KAb201, an anti-carcinoembryonic antigen monoclonal antibody, labelled with I(131) in pancreatic cancer (ISRCTN 16857581).
Methods: Patients with histological/cytological proven inoperable adenocarcinoma of the head of pancreas were randomised to receive KAb 201 via either the intra-arterial or intravenous delivery route. The dose limiting toxicities within each group were determined. Patients were assessed for safety and efficacy and followed up until death.
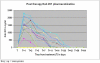
Results: Between February 2003 and July 2005, 25 patients were enrolled. Nineteen patients were randomised, 9 to the intravenous and 10 to the intra-arterial arms. In the intra-arterial arm, dose limiting toxicity was seen in 2/6 (33%) patients at 50 mCi whereas in the intravenous arm, dose limiting toxicity was noted in 1/6 patients at 50 mCi, but did not occur at 75 mCi (0/3).The overall response rate was 6% (1/18). Median overall survival was 5.2 months (95% confidence interval = 3.3 to 9 months), with no significant difference between the intravenous and intra-arterial arms (log rank test p = 0.79). One patient was still alive at the time of this analysis.
Conclusion: Dose limiting toxicity for KAb201 with I(131) by the intra-arterial route was 50 mCi, while dose limiting toxicity was not reached in the intravenous arm.
Figures



References
-
- Cancer Stats Incidence. http://info.cancerresearchuk.org/cancerstats
-
- Cancer Stat Fact Sheet- Cancer of the pancreas. http://seer.cancer.gov/statfacts/html/pancreas.html
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

