Characterization of the neurohypophysial hormone gene loci in elephant shark and the Japanese lamprey: origin of the vertebrate neurohypophysial hormone genes
- PMID: 19243634
- PMCID: PMC2656470
- DOI: 10.1186/1471-2148-9-47
Characterization of the neurohypophysial hormone gene loci in elephant shark and the Japanese lamprey: origin of the vertebrate neurohypophysial hormone genes
Abstract
Background: Vasopressin and oxytocin are mammalian neurohypophysial hormones with distinct functions. Vasopressin is involved mainly in osmoregulation and oxytocin is involved primarily in parturition and lactation. Jawed vertebrates contain at least one homolog each of vasopressin and oxytocin, whereas only a vasopressin-family hormone, vasotocin, has been identified in jawless vertebrates. The genes encoding vasopressin and oxytocin are closely linked tail-to-tail in eutherian mammals whereas their homologs in chicken, Xenopus and coelacanth (vasotocin and mesotocin) are linked tail-to-head. In contrast, their pufferfish homologs, vasotocin and isotocin, are located on the same strand of DNA with isotocin located upstream of vasotocin and separated by five genes. These differences in the arrangement of the two genes in different bony vertebrate lineages raise questions about their origin and ancestral arrangement. To trace the origin of these genes, we have sequenced BAC clones from the neurohypophysial gene loci in a cartilaginous fish, the elephant shark (Callorhinchus milii), and in a jawless vertebrate, the Japanese lamprey (Lethenteron japonicum). We have also analyzed the neurohypophysial hormone gene locus in an invertebrate chordate, the amphioxus (Branchiostoma floridae).
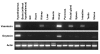
Results: The elephant shark neurohypophysial hormone genes encode vasotocin and oxytocin, and are linked tail-to-head like their homologs in coelacanth and non-eutherian tetrapods. Besides the hypothalamus, the two genes are also expressed in the ovary. In addition, the vasotocin gene is expressed in the kidney, rectal gland and intestine. These expression profiles indicate a paracrine role for the two hormones. The lamprey locus contains a single neurohypophysial hormone gene, the vasotocin. The synteny of genes in the lamprey locus is conserved in elephant shark, coelacanth and tetrapods but disrupted in teleost fishes. The amphioxus locus encodes a single neurohypophysial hormone, designated as [Ile4]vasotocin.
Conclusion: The vasopressin- and oxytocin-family of neurohypophysial hormones evolved in a common ancestor of jawed vertebrates through tandem duplication of the ancestral vasotocin gene. The duplicated genes were linked tail-to-head like their homologs in elephant shark, coelacanth and non-eutherian tetrapods. In contrast to the conserved linkage of the neurohypophysial genes in these vertebrates, the neurohypophysial hormone gene locus has experienced extensive rearrangements in the teleost lineage.
Figures






References
-
- Suzuki M, Kubokawa K, Nagasawa H, Urano A. Sequence analysis of vasotocin cDNAs of the lamprey, Lampetra japonica, and the hagfish, Eptatretus burgeri: evolution of cyclostome vasotocin precursors. J Mol Endocrinol. 1995;14:67–77. - PubMed
-
- Lane TF, Sower SA, Kawauchi H. Arginine vasotocin from the pituitary gland of the lamprey (Petromyzon marinus): isolation and amino acid sequence. Gen Comp Endocrinol. 1988;70:152–157. - PubMed
-
- Mohr E, Bahnsen U, Kiessling C, Richter D. Expression of the vasopressin and oxytocin genes in rats occurs in mutually exclusive sets of hypothalamic neurons. FEBS Lett. 1988;242:144–148. - PubMed
-
- Davies J, Waller S, Zeng Q, Wells S, Murphy D. Further delineation of the sequences required for the expression and physiological regulation of the vasopressin gene in transgenic rat hypothalamic magnocellular neurones. J Neuroendocrinol. 2003;15:42–50. - PubMed
-
- Murphy D, Wells S. In vivo gene transfer studies on the regulation and function of the vasopressin and oxytocin genes. J Neuroendocrinol. 2003;15:109–125. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

