Biodistributions, myelosuppression, and toxicities in mice treated with an anti-CD45 antibody labeled with the alpha-emitting radionuclides bismuth-213 or astatine-211
- PMID: 19244101
- PMCID: PMC2657815
- DOI: 10.1158/0008-5472.CAN-08-4363
Biodistributions, myelosuppression, and toxicities in mice treated with an anti-CD45 antibody labeled with the alpha-emitting radionuclides bismuth-213 or astatine-211
Abstract
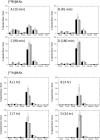
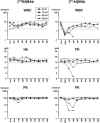
We previously investigated the potential of targeted radiotherapy using a bismuth-213 ((213)Bi)-labeled anti-CD45 antibody to replace total body irradiation as conditioning for hematopoietic cell transplantation in a canine model. Although this approach allowed sustained marrow engraftment, limited availability, high cost, and short half-life of (213)Bi induced us to investigate an alternative alpha-emitting radionuclide, astatine-211 ((211)At), for the same application. Biodistribution and toxicity studies were conducted with conjugates of the anti-murine CD45 antibody 30F11 with either (213)Bi or (211)At. Mice were injected with 2 to 50 muCi on 10 microg or 20 muCi on 2 or 40 microg of 30F11 conjugate. Biodistribution studies showed that the spleen contained the highest concentration of radioactivity, ranging from 167 +/- 23% to 417 +/- 109% injected dose/gram (% ID/g) after injection of the (211)At conjugate and 45 +/- 9% to 166 +/- 11% ID/g after injection of the (213)Bi conjugate. The higher concentrations observed for (211)At-labeled 30F11 were due to its longer half-life, which permitted better localization of isotope to the spleen before decay. (211)At was more effective at producing myelosuppression for the same quantity of injected radioactivity. All mice injected with 20 or 50 muCi (211)At, but none with the same quantities of (213)Bi, had lethal myeloablation. Severe reversible acute hepatic toxicity occurred with 50 muCi (213)Bi, but not with lower doses of (213)Bi or with any dose of (211)At. No renal toxicity occurred with either radionuclide. The data suggest that smaller quantities of (211)At-labeled anti-CD45 antibody are sufficient to achieve myelosuppression and myeloablation with less nonhematologic toxicity compared with (213)Bi-labeled antibody.
Figures


References
-
- Matthews DC, Appelbaum FR, Eary JF, et al. Development of a marrow transplant regimen for acute leukemia using targeted hematopoietic irradiation delivered by 131I-labeled anti-CD45 antibody, combined with cyclophosphamide and total body irradiation. Blood. 1995;85:1122–31. - PubMed
-
- Matthews DC, Martin PJ, Nourigat C, Appelbaum FR, Fisher DR, Bernstein ID. Marrow ablative and immunosuppressive effects of 131 I-anti-CD45 antibody in congenic and H2-mismatched murine transplant models. Blood. 1999;93:737–45. - PubMed
-
- Matthews DC, Appelbaum FR, Eary JF, et al. Phase I study of 131I-Anti-CD45 antibody plus cyclophosphamide and total body irradiation for advanced acute leukemia and myelodysplastic syndrome. Blood. 1999;94:1237–47. - PubMed
-
- Fisher DR. Alpha-particle emitters in medicine. In: Adelstein SJ, Kassis AI, Burt RW, editors. Dosimetry of Administered Radionucles. The American College of Nuclear Physicians; Washington, DC: 1989. pp. 194–214.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

