The long lifespan of two bat species is correlated with resistance to protein oxidation and enhanced protein homeostasis
- PMID: 19244163
- PMCID: PMC2704589
- DOI: 10.1096/fj.08-122523
The long lifespan of two bat species is correlated with resistance to protein oxidation and enhanced protein homeostasis
Abstract
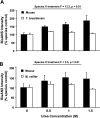
Altered structure, and hence function, of cellular macromolecules caused by oxidation can contribute to loss of physiological function with age. Here, we tested whether the lifespan of bats, which generally live far longer than predicted by their size, could be explained by reduced protein damage relative to short-lived mice. We show significantly lower protein oxidation (carbonylation) in Mexican free-tailed bats (Tadarida brasiliensis) relative to mice, and a trend for lower oxidation in samples from cave myotis bats (Myotis velifer) relative to mice. Both species of bat show in vivo and in vitro resistance to protein oxidation under conditions of acute oxidative stress. These bat species also show low levels of protein ubiquitination in total protein lysates along with reduced proteasome activity, suggesting diminished protein damage and removal in bats. Lastly, we show that bat-derived protein fractions are resistant to urea-induced protein unfolding relative to the level of unfolding detected in fractions from mice. Together, these data suggest that long lifespan in some bat species might be regulated by very efficient maintenance of protein homeostasis.
Figures





References
-
- Finch C E. Chicago: University of Chicago Press; Longevity, Senescence and the Genome. 1990
-
- George J C, Bada J, Zeh J, Scott L, Brown S E, O'Hara T, Suydam R. Age and growth estimates of bowhead whales (Balaena mysticetus) via aspartic acid racemization. Can J Zool. 1999;77:571–580.
-
- Austad S N, Fischer K E. Mammalian aging, metabolism, and ecology: evidence from the bats and marsupials. J Gerontol A Biol Sci Med Sci. 1991;46:B47–B53. - PubMed
-
- Wilkinson G S, South J M. Life history, ecology and longevity in bats. Aging Cell. 2002;1:124–131. - PubMed
-
- Jürgens K D, Prothero J. Scaling of maximal lifespan in bats. Comp Biochem Physiol. 1987;88A:361–367. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

