Long-term effectiveness of adjuvant goserelin in premenopausal women with early breast cancer
- PMID: 19244174
- PMCID: PMC2650713
- DOI: 10.1093/jnci/djn498
Long-term effectiveness of adjuvant goserelin in premenopausal women with early breast cancer
Abstract
Background: Systematic reviews have found that luteinizing hormone-releasing hormone (LHRH) agonists are effective in treating premenopausal women with early breast cancer.
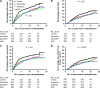
Methods: We conducted long-term follow-up (median 12 years) of 2706 women in the Zoladex In Premenopausal Patients (ZIPP), which evaluated the LHRH agonist goserelin (3.6 mg injection every 4 weeks) and tamoxifen (20 or 40 mg daily), given for 2 years. Women were randomly assigned to receive each therapy alone, both, or neither, after primary therapy (surgery with or without radiotherapy/chemotherapy). Hazard ratios and absolute risk differences were used to assess the effect of goserelin treatment on event-free survival (breast cancer recurrence, new tumor or death), overall survival, risk of recurrence of breast cancer, and risk of dying from breast cancer, in the presence or absence of tamoxifen.
Results: Fifteen years after the initiation of treatment, for every 100 women not given tamoxifen, there were 13.9 (95% confidence interval [CI] = 17.5 to 19.4) fewer events among those who were treated with goserelin compared with those who were not treated with goserelin. However, among women who did take tamoxifen, there were 2.8 fewer events (95% CI = 7.7 fewer to 2.0 more) per 100 women treated with goserelin compared with those not treated with goserelin. The risk of dying from breast cancer was also reduced at 15 years: For every 100 women given goserelin, the number of breast cancer deaths was lower by 2.6 (95% CI = 6.6 fewer to 2.1 more) and 8.5 (95% CI = 2.2 to 13.7) in those who did and did not take tamoxifen, respectively, although in the former group the difference was not statistically significant.
Conclusions: Two years of goserelin treatment was as effective as 2 years of tamoxifen treatment 15 years after starting therapy. In women who did not take tamoxifen, there was a large benefit of goserelin treatment on survival and recurrence, and in women who did take tamoxifen, there was a marginal potential benefit on these outcomes when goserelin was added.
Figures


References
-
- Kelsey JL, Bernstein L. Epidemiology and prevention of breast cancer. Annu Rev Public Health. 1996;17(1):47–67. - PubMed
-
- Hankinson SE, Willett WC, Colditz GA, et al. Circulating concentrations of insulin-like growth factor-I and risk of breast cancer. Lancet. 1998;351(9113):1393–1396. - PubMed
-
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–1717. - PubMed
-
- LHRH-agonists in Early Breast Cancer Overview Group. Use of luteinising hormone-releasing hormone (LHRH) agonists as adjuvant treatment in premenopausal patients with hormone-receptor-positive breast cancer: a meta-analysis of individual patient data from randomised adjuvant trials. Lancet. 2007;369(9574):1711–1723. - PubMed
-
- Baum M, Hackshaw A, Houghton J, et al. Adjuvant goserelin in premenopausal patients with early breast cancer: results from the ‘Zoladex’ in premenopausal patients (ZIPP) trial. Eur J Cancer. 2006;42(7):895–904. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

