Wnt-5a-CKI{alpha} signaling promotes {beta}-catenin/E-cadherin complex formation and intercellular adhesion in human breast epithelial cells
- PMID: 19244247
- PMCID: PMC2667782
- DOI: 10.1074/jbc.M804923200
Wnt-5a-CKI{alpha} signaling promotes {beta}-catenin/E-cadherin complex formation and intercellular adhesion in human breast epithelial cells
Abstract
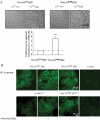
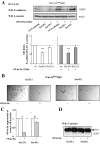
Wnt-5a is a non-transforming Wnt protein that is implicated in cell polarity, adhesion, and motility. We have previously shown that low expression of Wnt-5a is a predictor of shorter disease-free survival in human breast cancer. Here, we investigated whether beta-catenin/E-cadherin-mediated cell-cell adhesion was affected by loss of Wnt-5a in breast carcinomas, thereby promoting a metastatic behavior of the tumor. We show that Wnt-5a stimulation of human breast epithelial cells leads to an increased Ca(2+)-dependent cell-cell adhesion. Furthermore, Wnt-5a/casein kinase Ialpha (CKIalpha)-specific Ser-45 phosphorylation of beta-catenin is associated with an increased complex formation of beta-catenin/E-cadherin. Mutation of Ser-45 decreases the beta-catenin/E-cadherin association. Also, the inhibitory effect of Wnt-5a on breast epithelial cell invasion is reduced upon mutation of beta-catenin-Ser-45. The Wnt-5a-CKIalpha-induced Ser-45 phosphorylation does not lead to degradation of beta-catenin. Finally we show that human breast cancers lacking Wnt-5a protein have a significantly lower level of membrane-associated beta-catenin. Down-regulation of Wnt-5a expression and subsequent reduction of membrane-associated beta-catenin in invasive breast cancer, can therefore contribute to a decreased cell-cell adhesion and increased motility resulting in a higher probability for metastatic disease.
Figures






References
-
- Osborne, M. P. (2000) Breast Anatomy and Development, 2nd Ed., Philadelphia, PA
-
- Berx, G., Nollet, F., and van Roy, F. (1998) Cell Adhes. Commun. 6 171-184 - PubMed
-
- Lei, H., Sjoberg-Margolin, S., Salahshor, S., Werelius, B., Jandakova, E., Hemminki, K., Lindblom, A., and Vorechovsky, I. (2002) Int. J. Cancer 98 199-204 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

