Role of conserved histidine residues in the low-pH dependence of the Semliki Forest virus fusion protein
- PMID: 19244325
- PMCID: PMC2668505
- DOI: 10.1128/JVI.02646-08
Role of conserved histidine residues in the low-pH dependence of the Semliki Forest virus fusion protein
Abstract
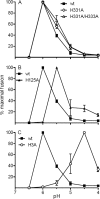
A wide variety of enveloped viruses infects cells by taking advantage of the low pH in the endocytic pathway to trigger virus-membrane fusion. For alphaviruses such as Semliki Forest virus (SFV), acidic pH initiates a series of conformational changes in the heterodimeric virus envelope proteins E1 and E2. Low pH dissociates the E2/E1 dimer, releasing the membrane fusion protein E1. E1 inserts into the target membrane and refolds to a trimeric hairpin conformation, thus driving the fusion reaction. The means by which E1 senses and responds to low pH is unclear, and protonation of conserved E1 histidine residues has been proposed as a possible mechanism. We tested the role of four conserved histidines by mutagenesis of the wild-type (wt) SFV infectious clone to create virus mutants with E1 H3A, H125A, H331A, and H331A/H333A mutations. The H125A, H331A, and H331A/H333A mutants had growth properties similar to those of wt SFV and showed modest change or no change in the pH dependence of virus-membrane fusion. By contrast, the E1 H3A mutation produced impaired virus growth and a markedly more acidic pH requirement for virus-membrane fusion. The dissociation of the H3A heterodimer and the membrane insertion of the mutant E1 protein were comparable to those of the wt in efficiency and pH dependence. However, the formation of the H3A homotrimer required a much lower pH and showed reduced efficiency. Together, these results and the location of H3 suggest that this residue acts to regulate the low-pH-dependent refolding of E1 during membrane fusion.
Figures



Similar articles
-
The domain I-domain III linker plays an important role in the fusogenic conformational change of the alphavirus membrane fusion protein.J Virol. 2011 Jul;85(13):6334-42. doi: 10.1128/JVI.00596-11. Epub 2011 May 4. J Virol. 2011. PMID: 21543498 Free PMC article.
-
E1 mutants identify a critical region in the trimer interface of the Semliki forest virus fusion protein.J Virol. 2009 Nov;83(21):11298-306. doi: 10.1128/JVI.01147-09. Epub 2009 Aug 19. J Virol. 2009. PMID: 19692469 Free PMC article.
-
Pseudorevertants of a Semliki forest virus fusion-blocking mutation reveal a critical interchain interaction in the core trimer.J Virol. 2010 Nov;84(22):11624-33. doi: 10.1128/JVI.01625-10. Epub 2010 Sep 8. J Virol. 2010. PMID: 20826687 Free PMC article.
-
Assembly and entry mechanisms of Semliki Forest virus.Arch Virol Suppl. 1994;9:329-38. doi: 10.1007/978-3-7091-9326-6_33. Arch Virol Suppl. 1994. PMID: 8032265 Review.
-
Viral inactivation based on inhibition of membrane fusion: understanding the role of histidine protonation to develop new viral vaccines.Protein Pept Lett. 2009;16(7):779-85. doi: 10.2174/092986609788681823. Protein Pept Lett. 2009. PMID: 19601907 Review.
Cited by
-
Localization of a region in the fusion protein of avian metapneumovirus that modulates cell-cell fusion.J Virol. 2012 Nov;86(21):11800-14. doi: 10.1128/JVI.00232-12. Epub 2012 Aug 22. J Virol. 2012. PMID: 22915815 Free PMC article.
-
Protonation of individual histidine residues is not required for the pH-dependent entry of west nile virus: evaluation of the "histidine switch" hypothesis.J Virol. 2009 Dec;83(23):12631-5. doi: 10.1128/JVI.01072-09. Epub 2009 Sep 23. J Virol. 2009. PMID: 19776132 Free PMC article.
-
Studies of the "chain reversal regions" of the avian sarcoma/leukosis virus (ASLV) and ebolavirus fusion proteins: analogous residues are important, and a His residue unique to EnvA affects the pH dependence of ASLV entry.J Virol. 2010 Jun;84(11):5687-94. doi: 10.1128/JVI.02583-09. Epub 2010 Mar 24. J Virol. 2010. PMID: 20335266 Free PMC article.
-
Marburg virus glycoprotein GP2: pH-dependent stability of the ectodomain α-helical bundle.Biochemistry. 2012 Mar 27;51(12):2515-25. doi: 10.1021/bi3000353. Epub 2012 Mar 12. Biochemistry. 2012. PMID: 22369502 Free PMC article.
-
A tyrosine-to-histidine switch at position 18 of the Ross River virus E2 glycoprotein is a determinant of virus fitness in disparate hosts.J Virol. 2013 May;87(10):5970-84. doi: 10.1128/JVI.03326-12. Epub 2013 Mar 20. J Virol. 2013. PMID: 23514884 Free PMC article.
References
-
- Carneiro, F. A., F. Stauffer, C. S. Lima, M. A. Juliano, L. Juliano, and A. T. Da Poian. 2003. Membrane fusion induced by vesicular stomatitis virus depends on histidine protonation. J. Biol. Chem. 27813789-13794. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

