Function and regulation of TRPP2 at the plasma membrane
- PMID: 19244406
- PMCID: PMC2711710
- DOI: 10.1152/ajprenal.90277.2008
Function and regulation of TRPP2 at the plasma membrane
Abstract
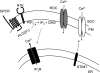
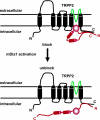
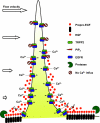
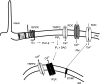
The vast majority (approximately 99%) of all known cases of autosomal dominant polycystic kidney disease (ADPKD) are caused by naturally occurring mutations in two separate, but genetically interacting, loci, pkd1 and pkd2. pkd1 encodes a large multispanning membrane protein (PKD1) of unknown function, while pkd2 encodes a protein (TRPP2, polycystin-2, or PKD2) of the transient receptor potential (TRP) superfamily of ion channels. Biochemical, functional, and genetic studies support a model in which PKD1 physically interacts with TRPP2 to form an ion channel complex that conveys extracellular stimuli to ionic currents. However, the molecular identity of these extracellular stimuli remains elusive. Functional studies in cell culture show that TRPP2 can be activated in response to mechanical cues (fluid shear stress) and/or receptor tyrosine kinase (RTK) and G protein-coupled receptor (GPCR) activation at the cell surface. Recent genetic studies in Chlamydomonas reinhardtii show that CrPKD2 functions in a pathway linking cell-cell adhesion and Ca(2+) signaling. The mode of activation depends on protein-protein interactions with other channel subunits and auxiliary proteins. Therefore, understanding the mechanisms underlying the molecular makeup of TRPP2-containing complexes is critical in delineating the mechanisms of TRPP2 activation and, most importantly, the mechanisms by which naturally occurring mutations in pkd1 or pkd2 lead not only to ADPKD, but also to other defects reported in model organisms lacking functional TRPP2. This review focuses on the molecular assembly, function, and regulation of TRPP2 as a cell surface cation channel and discusses its potential role in Ca(2+) signaling and ADPKD pathophysiology.
Figures


Similar articles
-
Extracellular Loops Are Essential for the Assembly and Function of Polycystin Receptor-Ion Channel Complexes.J Biol Chem. 2017 Mar 10;292(10):4210-4221. doi: 10.1074/jbc.M116.767897. Epub 2017 Feb 2. J Biol Chem. 2017. PMID: 28154010 Free PMC article.
-
Function and regulation of TRPP2 ion channel revealed by a gain-of-function mutant.Proc Natl Acad Sci U S A. 2016 Apr 26;113(17):E2363-72. doi: 10.1073/pnas.1517066113. Epub 2016 Apr 11. Proc Natl Acad Sci U S A. 2016. PMID: 27071085 Free PMC article.
-
Polycystin-2 (TRPP2): Ion channel properties and regulation.Gene. 2022 Jun 15;827:146313. doi: 10.1016/j.gene.2022.146313. Epub 2022 Mar 18. Gene. 2022. PMID: 35314260 Review.
-
TRPP2 dysfunction decreases ATP-evoked calcium, induces cell aggregation and stimulates proliferation in T lymphocytes.BMC Nephrol. 2019 Sep 13;20(1):355. doi: 10.1186/s12882-019-1540-6. BMC Nephrol. 2019. PMID: 31514750 Free PMC article.
-
Polycystins as components of large multiprotein complexes of polycystin interactors.Cell Signal. 2020 Aug;72:109640. doi: 10.1016/j.cellsig.2020.109640. Epub 2020 Apr 17. Cell Signal. 2020. PMID: 32305669 Free PMC article. Review.
Cited by
-
Thermotaxis, circadian rhythms, and TRP channels in Drosophila.Temperature (Austin). 2015 Feb 11;2(2):227-43. doi: 10.1080/23328940.2015.1004972. eCollection 2015 Apr-Jun. Temperature (Austin). 2015. PMID: 27227026 Free PMC article. Review.
-
Calcium transport and local pool regulate polycystin-2 (TRPP2) function in human syncytiotrophoblast.Biophys J. 2013 Jul 16;105(2):365-75. doi: 10.1016/j.bpj.2013.05.058. Biophys J. 2013. PMID: 23870258 Free PMC article.
-
TRP Channels as Molecular Targets to Relieve Endocrine-Related Diseases.Front Mol Biosci. 2022 Apr 28;9:895814. doi: 10.3389/fmolb.2022.895814. eCollection 2022. Front Mol Biosci. 2022. PMID: 35573736 Free PMC article. Review.
-
Store-operated channels regulate intracellular calcium in mammalian rods.J Physiol. 2012 Aug 1;590(15):3465-81. doi: 10.1113/jphysiol.2012.234641. Epub 2012 Jun 6. J Physiol. 2012. PMID: 22674725 Free PMC article.
-
Non-voltage-gated Ca2+ channel signaling in glomerular cells in kidney health and disease.Am J Physiol Renal Physiol. 2024 Aug 1;327(2):F249-F264. doi: 10.1152/ajprenal.00130.2024. Epub 2024 Jun 13. Am J Physiol Renal Physiol. 2024. PMID: 38867675 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

