State-dependent block of HERG potassium channels by R-roscovitine: implications for cancer therapy
- PMID: 19244476
- PMCID: PMC2670659
- DOI: 10.1152/ajpcell.00633.2008
State-dependent block of HERG potassium channels by R-roscovitine: implications for cancer therapy
Abstract
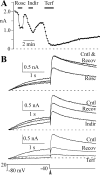
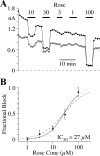
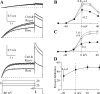
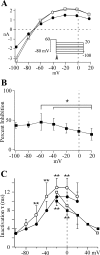
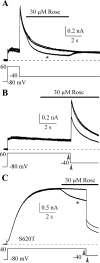
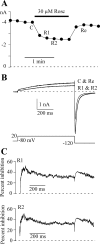
Human ether-a-go-go-related gene (HERG) potassium channel acts as a delayed rectifier in cardiac myocytes and is an important target for both pro- and antiarrhythmic drugs. Many drugs have been pulled from the market for unintended HERG block causing arrhythmias. Conversely, recent evidence has shown that HERG plays a role in cell proliferation and is overexpressed both in multiple tumor cell lines and in primary tumor cells, which makes HERG an attractive target for cancer treatment. Therefore, a drug that can block HERG but that does not induce cardiac arrhythmias would have great therapeutic potential. Roscovitine is a cyclin-dependent kinase (CDK) inhibitor that is in phase II clinical trials as an anticancer agent. In the present study we show that R-roscovitine blocks HERG potassium current (human embryonic kidney-293 cells stably expressing HERG) at clinically relevant concentrations. The block (IC(50) = 27 microM) was rapid (tau = 20 ms) and reversible (tau = 25 ms) and increased with channel activation, which supports an open channel mechanism. Kinetic study of wild-type and inactivation mutant HERG channels supported block of activated channels by roscovitine with relatively little effect on either closed or inactivated channels. A HERG gating model reproduced all roscovitine effects. Our model of open channel block by roscovitine may offer an explanation of the lack of arrhythmias in clinical trials using roscovitine, which suggests the utility of a dual CDK/HERG channel block as an adjuvant cancer therapy.
Figures


References
-
- Arcangeli A Expression and role of hERG channels in cancer cells. Novartis Found Symp 266: 225–232; discussion 232–224, 2005. - PubMed
-
- Benson C, White J, De Bono J, O'Donnell A, Raynaud F, Cruickshank C, McGrath H, Walton M, Workman P, Kaye S, Cassidy J, Gianella-Borradori A, Judson I, Twelves C. A phase I trial of the selective oral cyclin-dependent kinase inhibitor seliciclib (CYC202; R-roscovitine), administered twice daily for 7 days every 21 days. Br J Cancer 96: 29–37, 2007. - PMC - PubMed
-
- Bril A, Gout B, Bonhomme M, Landais L, Faivre JF, Linee P, Poyser RH, Ruffolo RR Jr. Combined potassium and calcium channel blocking activities as a basis for antiarrhythmic efficacy with low proarrhythmic risk: experimental profile of BRL-32872. J Pharmacol Exp Ther 276: 637–646, 1996. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

