Age-dependent alterations of corticostriatal activity in the YAC128 mouse model of Huntington disease
- PMID: 19244517
- PMCID: PMC2670193
- DOI: 10.1523/JNEUROSCI.5687-08.2009
Age-dependent alterations of corticostriatal activity in the YAC128 mouse model of Huntington disease
Abstract
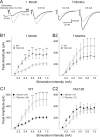
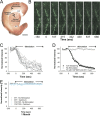
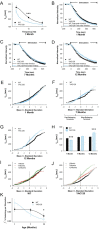
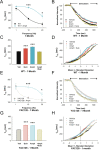
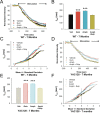
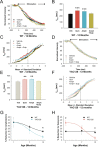
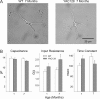
Huntington disease is a genetic neurodegenerative disorder that produces motor, neuropsychiatric, and cognitive deficits and is caused by an abnormal expansion of the CAG tract in the huntingtin (htt) gene. In humans, mutated htt induces a preferential loss of medium spiny neurons in the striatum and, to a lesser extent, a loss of cortical neurons as the disease progresses. The mechanisms causing these degenerative changes remain unclear, but they may involve synaptic dysregulation. We examined the activity of the corticostriatal pathway using a combination of electrophysiological and optical imaging approaches in brain slices and acutely dissociated neurons from the YAC128 mouse model of Huntington disease. The results demonstrated biphasic age-dependent changes in corticostriatal function. At 1 month, before the behavioral phenotype develops, synaptic currents and glutamate release were increased. At 7 and 12 months, after the development of the behavioral phenotype, evoked synaptic currents were reduced. Glutamate release was decreased by 7 months and was markedly reduced by 12 months. These age-dependent alterations in corticostriatal activity were paralleled by a decrease in dopamine D(2) receptor modulation of the presynaptic terminal. Together, these findings point to dynamic alterations at the corticostriatal pathway and emphasize that therapies directed toward preventing or alleviating symptoms need to be specifically designed depending on the stage of disease progression.
Figures

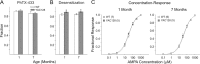
Similar articles
-
Differential changes in thalamic and cortical excitatory synapses onto striatal spiny projection neurons in a Huntington disease mouse model.Neurobiol Dis. 2016 Feb;86:62-74. doi: 10.1016/j.nbd.2015.11.020. Epub 2015 Nov 24. Neurobiol Dis. 2016. PMID: 26621114
-
Transient and progressive electrophysiological alterations in the corticostriatal pathway in a mouse model of Huntington's disease.J Neurosci. 2003 Feb 1;23(3):961-9. doi: 10.1523/JNEUROSCI.23-03-00961.2003. J Neurosci. 2003. PMID: 12574425 Free PMC article.
-
Impaired development of cortico-striatal synaptic connectivity in a cell culture model of Huntington's disease.Neurobiol Dis. 2016 Mar;87:80-90. doi: 10.1016/j.nbd.2015.12.009. Epub 2015 Dec 19. Neurobiol Dis. 2016. PMID: 26711622
-
The corticostriatal pathway in Huntington's disease.Prog Neurobiol. 2007 Apr;81(5-6):253-71. doi: 10.1016/j.pneurobio.2006.11.001. Epub 2006 Dec 13. Prog Neurobiol. 2007. PMID: 17169479 Free PMC article. Review.
-
The selective vulnerability of nerve cells in Huntington's disease.Neuropathol Appl Neurobiol. 2001 Feb;27(1):1-21. doi: 10.1046/j.0305-1846.2001.00299.x. Neuropathol Appl Neurobiol. 2001. PMID: 11298997 Review.
Cited by
-
Real-time imaging of glutamate clearance reveals normal striatal uptake in Huntington disease mouse models.Nat Commun. 2016 Apr 7;7:11251. doi: 10.1038/ncomms11251. Nat Commun. 2016. PMID: 27052848 Free PMC article.
-
Genetics and neuropathology of Huntington's disease.Int Rev Neurobiol. 2011;98:325-72. doi: 10.1016/B978-0-12-381328-2.00014-6. Int Rev Neurobiol. 2011. PMID: 21907094 Free PMC article. Review.
-
Altering cortical input unmasks synaptic phenotypes in the YAC128 cortico-striatal co-culture model of Huntington disease.BMC Biol. 2018 Jun 27;16(1):58. doi: 10.1186/s12915-018-0526-3. BMC Biol. 2018. PMID: 29945611 Free PMC article.
-
Unravelling and Exploiting Astrocyte Dysfunction in Huntington's Disease.Trends Neurosci. 2017 Jul;40(7):422-437. doi: 10.1016/j.tins.2017.05.002. Epub 2017 May 31. Trends Neurosci. 2017. PMID: 28578789 Free PMC article. Review.
-
Dopamine and glutamate in Huntington's disease: A balancing act.CNS Neurosci Ther. 2010 Jun;16(3):163-78. doi: 10.1111/j.1755-5949.2010.00134.x. Epub 2010 Apr 8. CNS Neurosci Ther. 2010. PMID: 20406248 Free PMC article. Review.
References
-
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. - PubMed
-
- Albin RL, Reiner A, Anderson KD, Penney JB, Young AB. Striatal and nigral neuron subpopulations in rigid Huntington's disease: implications for the functional anatomy of chorea and rigidity-akinesia. Ann Neurol. 1990;27:357–365. - PubMed
-
- André VM, Cepeda C, Venegas A, Gomez Y, Levine MS. Altered cortical glutamate receptor function in the R6/2 model of Huntington's disease. J Neurophysiol. 2006;95:2108–2119. - PubMed
-
- Ariano MA, Aronin N, Difiglia M, Tagle DA, Sibley DR, Leavitt BR, Hayden MR, Levine MS. Striatal neurochemical changes in transgenic models of Huntington's disease. J Neurosci Res. 2002;68:716–729. - PubMed
-
- Ariano MA, Cepeda C, Calvert CR, Flores-Hernández J, Hernández-Echeagaray E, Klapstein GJ, Chandler SH, Aronin N, DiFiglia M, Levine MS. Striatal potassium channel dysfunction in Huntington's disease transgenic mice. J Neurophysiol. 2005;93:2565–2574. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
