The BOLD response in the rat hippocampus depends rather on local processing of signals than on the input or output activity. A combined functional MRI and electrophysiological study
- PMID: 19244518
- PMCID: PMC6666263
- DOI: 10.1523/JNEUROSCI.5015-08.2009
The BOLD response in the rat hippocampus depends rather on local processing of signals than on the input or output activity. A combined functional MRI and electrophysiological study
Abstract
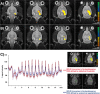
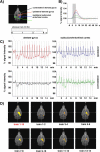
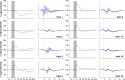
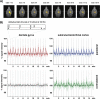
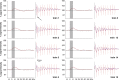
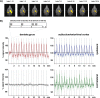
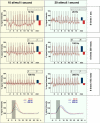
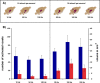
Functional magnetic resonance imaging (fMRI) of the human brain is widely used in neuroscience, but the exact relationship between measured fMRI response and the underlying changes in neuronal activity is still elusive. To obtain further information about the specific roles of synaptic (input) and spiking activity (output) for the generation of fMRI-related signals, we used an approach that combines electrophysiological and MRI measurements in the anatomically and physiologically well defined rat hippocampus. Direct electrical stimulation of the perforant pathway enabled us to control synchronized input activity to the dentate gyrus, whereas recorded population spikes from the granular cell layer indicated the dentate output activity. The perforant pathway was first stimulated with 15 identical pulse trains (10 Hz for 8 s), and evoked blood oxygenation level-dependent (BOLD) responses and population spikes were measured for each individual stimulus train. Spatial and magnitude aspects of the elicited BOLD responses differentially changed in the dentate gyrus and hippocampus from early to late stimulus trains together with population spike latencies in the dentate indicating delayed inhibitory network processing. Furthermore, the same number of stimuli presented in different patterns within trains (i.e., bursts of 10 stimuli at 50, 100, or 200 Hz) clearly altered the BOLD responses. Similarly, variations in the BOLD response also occurred when different stimulus patterns were chosen that caused the same number of population spikes. The results indicate that neuronal network activity including inhibitory interneurons rather than exclusively the input or spiking activity of the principal neurons determine a BOLD response to repetitive stimuli.
Figures

References
-
- Amaral D, Lavenex P. Hippocampal neuroanatomy. In: Andersen P, Morris R, Amaral D, Bliss T, O'Keefe J, editors. The Hippocampus book. Oxford: Oxford UP; 2007. pp. 37–114.
-
- Angenstein F, Kammerer E, Niessen HG, Frey JU, Scheich H, Frey S. Frequency-dependent activation pattern in the rat hippocampus, a simultaneous electrophysiological and fMRI study. Neuroimage. 2007;38:150–163. - PubMed
-
- Buhl E, Whittington M. Local circuits. In: Andersen P, Morris R, Amaral D, Bliss T, O'Keefe J, editors. The hippocampus book. Oxford: Oxford UP; 2007. pp. 297–319.
-
- Harel N, Lee SP, Nagaoka T, Kim DS, Kim SG. Origin of negative blood oxygenation level-dependent fMRI signals. J Cereb Blood Flow Metab. 2002;22:908–917. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
