NMDA receptor activation increases free radical production through nitric oxide and NOX2
- PMID: 19244529
- PMCID: PMC2669930
- DOI: 10.1523/JNEUROSCI.0133-09.2009
NMDA receptor activation increases free radical production through nitric oxide and NOX2
Abstract
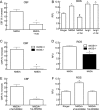
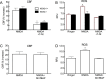
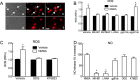
Reactive oxygen species (ROS) and nitric oxide (NO) participate in NMDA receptor signaling. However, the source(s) of the ROS and their role in the increase in cerebral blood flow (CBF) induced by NMDA receptor activation have not been firmly established. NADPH oxidase generates ROS in neurons, but there is no direct evidence that this enzyme is present in neurons containing NMDA receptors, or that is involved in NMDA receptor-dependent ROS production and CBF increase. We addressed these questions using a combination of in vivo and in vitro approaches. We found that the CBF and ROS increases elicited by topical application of NMDA to the mouse neocortex were both dependent on neuronal NO synthase (nNOS), cGMP, and the cGMP effector kinase protein kinase G (PKG). In mice lacking the NADPH oxidase subunit NOX2, the ROS increase was not observed, but the CBF increase was still present. Electron microscopy of the neocortex revealed NOX2 immunolabeling in postsynaptic somata and dendrites that also expressed the NMDA receptor NR1 subunit and nNOS. In neuronal cultures, the NMDA-induced increase in ROS was mediated by NADPH oxidase through NO, cGMP and PKG. We conclude that NADPH oxidase in postsynaptic neurons generates ROS during NMDA receptor activation. However, NMDA receptor-derived ROS do not contribute to the CBF increase. The findings establish a NOX2-containing NADPH oxidase as a major source of ROS produced by NMDA receptor activation, and identify NO as the critical link between NMDA receptor activity and NOX2-dependent ROS production.
Figures



Similar articles
-
Angiotensin II slow-pressor hypertension enhances NMDA currents and NOX2-dependent superoxide production in hypothalamic paraventricular neurons.Am J Physiol Regul Integr Comp Physiol. 2013 Jun 15;304(12):R1096-106. doi: 10.1152/ajpregu.00367.2012. Epub 2013 Apr 10. Am J Physiol Regul Integr Comp Physiol. 2013. PMID: 23576605 Free PMC article.
-
Chronic intermittent hypoxia induces NMDA receptor-dependent plasticity and suppresses nitric oxide signaling in the mouse hypothalamic paraventricular nucleus.J Neurosci. 2010 Sep 8;30(36):12103-12. doi: 10.1523/JNEUROSCI.3367-10.2010. J Neurosci. 2010. PMID: 20826673 Free PMC article.
-
NMDA receptor-nitric oxide transmission mediates neuronal iron homeostasis via the GTPase Dexras1.Neuron. 2006 Aug 17;51(4):431-40. doi: 10.1016/j.neuron.2006.07.011. Neuron. 2006. PMID: 16908409 Free PMC article.
-
Stop the flow: a paradigm for cell signaling mediated by reactive oxygen species in the pulmonary endothelium.Annu Rev Physiol. 2012;74:403-24. doi: 10.1146/annurev-physiol-020911-153324. Epub 2011 Nov 7. Annu Rev Physiol. 2012. PMID: 22077215 Free PMC article. Review.
-
Targeting NADPH oxidase and phospholipases A2 in Alzheimer's disease.Mol Neurobiol. 2010 Jun;41(2-3):73-86. doi: 10.1007/s12035-010-8107-7. Epub 2010 Mar 2. Mol Neurobiol. 2010. PMID: 20195796 Free PMC article. Review.
Cited by
-
Tau induces PSD95-neuronal NOS uncoupling and neurovascular dysfunction independent of neurodegeneration.Nat Neurosci. 2020 Sep;23(9):1079-1089. doi: 10.1038/s41593-020-0686-7. Epub 2020 Aug 10. Nat Neurosci. 2020. PMID: 32778793 Free PMC article.
-
The role of oxidative stress in organophosphate and nerve agent toxicity.Ann N Y Acad Sci. 2016 Aug;1378(1):17-24. doi: 10.1111/nyas.13115. Epub 2016 Jul 2. Ann N Y Acad Sci. 2016. PMID: 27371936 Free PMC article. Review.
-
Membrane trafficking of NADPH oxidase p47(phox) in paraventricular hypothalamic neurons parallels local free radical production in angiotensin II slow-pressor hypertension.J Neurosci. 2013 Mar 6;33(10):4308-16. doi: 10.1523/JNEUROSCI.3061-12.2013. J Neurosci. 2013. PMID: 23467347 Free PMC article.
-
Antioxidant and Neuroprotective Effects of N-((3,4-Dihydro-2H-benzo[h]chromen-2-yl)methyl)-4-methoxyaniline in Primary Cultured Rat Cortical Cells: Involvement of ERK-CREB Signaling.Molecules. 2018 Mar 15;23(3):669. doi: 10.3390/molecules23030669. Molecules. 2018. PMID: 29543778 Free PMC article.
-
Prohibitin viral gene transfer protects hippocampal CA1 neurons from ischemia and ameliorates postischemic hippocampal dysfunction.Stroke. 2014 Apr;45(4):1131-8. doi: 10.1161/STROKEAHA.113.003577. Epub 2014 Mar 11. Stroke. 2014. PMID: 24619393 Free PMC article.
References
-
- Anrather J, Racchumi G, Iadecola C. NF-kappa B regulates phagocytic NADPH oxidase by inducing the expression of gp91phox. J Biol Chem. 2006;281:5657–5667. - PubMed
-
- Atlante A, Gagliardi S, Minervini GM, Ciotti MT, Marra E, Calissano P. Glutamate neurotoxicity in rat cerebellar granule cells: a major role for xanthine oxidase in oxygen radical formation. J Neurochem. 1997;68:2038–2045. - PubMed
-
- Ayata C, Moskowitz MA. Cortical spreading depression confounds concentration-dependent pial arteriolar dilation during N-methyl-d-aspartate superfusion. Am J Physiol Heart Circ Physiol. 2006;290:H1837–H1841. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
