Deafness in TRbeta mutants is caused by malformation of the tectorial membrane
- PMID: 19244534
- PMCID: PMC2748340
- DOI: 10.1523/JNEUROSCI.3557-08.2009
Deafness in TRbeta mutants is caused by malformation of the tectorial membrane
Abstract
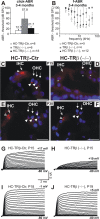
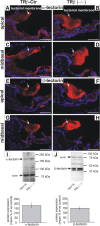
Thyroid hormone receptor beta (TRbeta) dysfunction leads to deafness in humans and mice. Deafness in TRbeta(-/-) mutant mice has been attributed to TRbeta-mediated control of voltage- and Ca(2+)-activated K(+) (BK) channel expression in inner hair cells (IHCs). However, normal hearing in young constitutive BKalpha(-/-) mutants contradicts this hypothesis. Here, we show that mice with hair cell-specific deletion of TRbeta after postnatal day 11 (P11) have a delay in BKalpha expression but normal hearing, indicating that the origin of hearing loss in TRbeta(-/-) mutant mice manifested before P11. Analyzing the phenotype of IHCs in constitutive TRbeta(-/-) mice, we found normal Ca(2+) current amplitudes, exocytosis, and shape of compound action potential waveforms. In contrast, reduced distortion product otoacoustic emissions and cochlear microphonics associated with an abnormal structure of the tectorial membrane and enhanced tectorin levels suggest that disturbed mechanical performance is the primary cause of deafness resulting from TRbeta deficiency.
Figures


Similar articles
-
Autonomous functions of murine thyroid hormone receptor TRα and TRβ in cochlear hair cells.Mol Cell Endocrinol. 2014 Jan 25;382(1):26-37. doi: 10.1016/j.mce.2013.08.025. Epub 2013 Sep 6. Mol Cell Endocrinol. 2014. PMID: 24012852
-
Deafness and permanently reduced potassium channel gene expression and function in hypothyroid Pit1dw mutants.J Neurosci. 2009 Jan 28;29(4):1212-23. doi: 10.1523/JNEUROSCI.4957-08.2009. J Neurosci. 2009. PMID: 19176829 Free PMC article.
-
Neuroplastin Isoform Np55 Is Expressed in the Stereocilia of Outer Hair Cells and Required for Normal Outer Hair Cell Function.J Neurosci. 2016 Aug 31;36(35):9201-16. doi: 10.1523/JNEUROSCI.0093-16.2016. J Neurosci. 2016. PMID: 27581460 Free PMC article.
-
The tectorial membrane: one slice of a complex cochlear sandwich.Curr Opin Otolaryngol Head Neck Surg. 2008 Oct;16(5):458-64. doi: 10.1097/MOO.0b013e32830e20c4. Curr Opin Otolaryngol Head Neck Surg. 2008. PMID: 18797289 Free PMC article. Review.
-
Hearing molecules: contributions from genetic deafness.Cell Mol Life Sci. 2007 Mar;64(5):566-80. doi: 10.1007/s00018-007-6417-3. Cell Mol Life Sci. 2007. PMID: 17260086 Free PMC article. Review.
Cited by
-
Thyroid hormone action in cerebellum and cerebral cortex development.J Thyroid Res. 2011;2011:145762. doi: 10.4061/2011/145762. Epub 2011 Jun 16. J Thyroid Res. 2011. PMID: 21765985 Free PMC article.
-
Deciphering direct and indirect influence of thyroid hormone with mouse genetics.Mol Endocrinol. 2014 Apr;28(4):429-41. doi: 10.1210/me.2013-1414. Epub 2014 Mar 10. Mol Endocrinol. 2014. PMID: 24617548 Free PMC article. Review.
-
Insights on the role of thyroid hormone transport in neurosensory organs and implication for the Allan-Herndon-Dudley syndrome.Eur Thyroid J. 2024 Mar 19;13(2):e230241. doi: 10.1530/ETJ-23-0241. Print 2024 Apr 1. Eur Thyroid J. 2024. PMID: 38417253 Free PMC article. Review.
-
Thyroid Hormone Receptor Beta in the Ventromedial Hypothalamus Is Essential for the Physiological Regulation of Food Intake and Body Weight.Cell Rep. 2017 Jun 13;19(11):2202-2209. doi: 10.1016/j.celrep.2017.05.066. Cell Rep. 2017. PMID: 28614708 Free PMC article.
-
Brown adipocytes local response to thyroid hormone is required for adaptive thermogenesis in adult male mice.Elife. 2022 Nov 14;11:e81996. doi: 10.7554/eLife.81996. Elife. 2022. PMID: 36374165 Free PMC article.
References
-
- Brucker-Davis F, Skarulis MC, Pikus A, Ishizawar D, Mastroianni MA, Koby M, Weintraub BD. Prevalence and mechanisms of hearing loss in patients with resistance to thyroid hormone. J Clin Endocrinol Metab. 1996;81:2768–2772. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous
