The neuropeptide pigment-dispersing factor adjusts period and phase of Drosophila's clock
- PMID: 19244536
- PMCID: PMC6666242
- DOI: 10.1523/JNEUROSCI.5439-08.2009
The neuropeptide pigment-dispersing factor adjusts period and phase of Drosophila's clock
Abstract
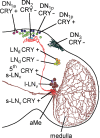
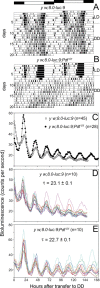
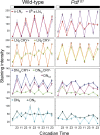
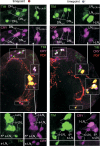
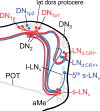
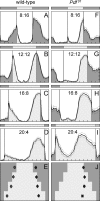
The neuropeptide pigment-dispersing factor (PDF) is a key transmitter in the circadian clock of Drosophila melanogaster. PDF is necessary for robust activity rhythms and is thought to couple the circadian oscillations of the clock neurons. However, little is known about the action of PDF on individual clock neurons. Here, we combined the period-luciferase reporter system with immunolabeling of clock proteins in wild-type and Pdf(01) mutants to dissect the effects of PDF on specific subgroups of clock neurons. Additionally, PDF levels were elevated to higher than normal levels using specific neural mutants, and a correlation analysis of locomotor activity and clock protein staining served to determine the periods of specific clock cells. We found that PDF has multiple effects on the clock neurons: In some groups of clock neurons, PDF was required for maintaining the oscillations of individual cells, and in others, PDF was required for synchronous cycling of the individual members. Other clock neurons cycled with high amplitude in absence of PDF, but PDF affected their intrinsic clock speed. Sometimes PDF shortened and sometimes PDF lengthened period. Our observations indicate that PDF is crucial for adjusting cycling amplitude, period, and phase of the different players in the circadian clock. Under natural conditions PDF may be required for adapting Drosophila's clock to varying photoperiods. Indeed, we show here that Pdf(01) mutants are not able to adapt their activity to long photoperiods in a wild-type manner.
Figures


Similar articles
-
Pigment-dispersing factor (PDF) has different effects on Drosophila's circadian clocks in the accessory medulla and in the dorsal brain.J Biol Rhythms. 2008 Oct;23(5):409-24. doi: 10.1177/0748730408322699. J Biol Rhythms. 2008. PMID: 18838607
-
The Neuropeptide PDF Is Crucial for Delaying the Phase of Drosophila's Evening Neurons Under Long Zeitgeber Periods.J Biol Rhythms. 2021 Oct;36(5):442-460. doi: 10.1177/07487304211032336. Epub 2021 Aug 24. J Biol Rhythms. 2021. PMID: 34428956 Free PMC article.
-
Disruption of synaptic transmission or clock-gene-product oscillations in circadian pacemaker cells of Drosophila cause abnormal behavioral rhythms.J Neurobiol. 2000 Jun 5;43(3):207-33. doi: 10.1002/(sici)1097-4695(20000605)43:3<207::aid-neu1>3.0.co;2-0. J Neurobiol. 2000. PMID: 10842235
-
Downloading central clock information in Drosophila.Mol Neurobiol. 2002 Oct-Dec;26(2-3):217-33. doi: 10.1385/MN:26:2-3:217. Mol Neurobiol. 2002. PMID: 12428757 Review.
-
Circadian clock genes in Drosophila: recent developments.Indian J Exp Biol. 2003 Aug;41(8):797-804. Indian J Exp Biol. 2003. PMID: 15248475 Review.
Cited by
-
Integration of Circadian Clock Information in the Drosophila Circadian Neuronal Network.J Biol Rhythms. 2021 Jun;36(3):203-220. doi: 10.1177/0748730421993953. Epub 2021 Mar 1. J Biol Rhythms. 2021. PMID: 33641476 Free PMC article. Review.
-
A new player in circadian networks: Role of electrical synapses in regulating functions of the circadian clock.Front Physiol. 2022 Nov 3;13:968574. doi: 10.3389/fphys.2022.968574. eCollection 2022. Front Physiol. 2022. PMID: 36406999 Free PMC article. Review.
-
Clock gene expression and locomotor activity predict death in the last days of life in Drosophila melanogaster.Sci Rep. 2018 Aug 9;8(1):11923. doi: 10.1038/s41598-018-30323-x. Sci Rep. 2018. PMID: 30093652 Free PMC article.
-
Dopamine and GPCR-mediated modulation of DN1 clock neurons gates the circadian timing of sleep.Proc Natl Acad Sci U S A. 2022 Aug 23;119(34):e2206066119. doi: 10.1073/pnas.2206066119. Epub 2022 Aug 15. Proc Natl Acad Sci U S A. 2022. PMID: 35969763 Free PMC article.
-
Light input pathways to the circadian clock of insects with an emphasis on the fruit fly Drosophila melanogaster.J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2020 Mar;206(2):259-272. doi: 10.1007/s00359-019-01379-5. Epub 2019 Nov 5. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2020. PMID: 31691095 Free PMC article. Review.
References
-
- Abrahamson EE, Moore RY. Suprachiasmatic nucleus in the mouse; retinal innervation, intrinsic organization and efferent projections. Brain Res. 2001;916:172–191. - PubMed
-
- Blanchardon E, Grima B, Klarsfeld A, Chélot E, Hardin PE, Préat T, Rouyer F. Defining the role of Drosophila lateral neurons in the control of circadian rhythms in motor activity and eclosion by targeted genetic ablation and PERIOD protein overexpression. Eur J Neurosci. 2001;13:871–888. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
