Effect of maternal nutrient restriction from early to midgestation on cardiac function and metabolism after adolescent-onset obesity
- PMID: 19244582
- PMCID: PMC2674931
- DOI: 10.1152/ajpregu.91019.2008
Effect of maternal nutrient restriction from early to midgestation on cardiac function and metabolism after adolescent-onset obesity
Abstract
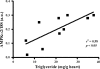
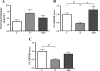
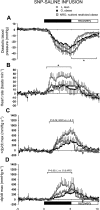
Maternal nutrient restriction (NR) from early to midgestation has marked effects on endocrine sensitivity and organ function of the resulting offspring. We hypothesized that early NR may reset the expression profile of genes central to myocardial energy metabolism, influencing ectopic lipid deposition and cardiac function in the obese adult offspring. NR offspring were exposed to an "obesogenic" environment, and their cardiac function and molecular indexes of myocardial energy metabolism were assessed to explore the hypothesis that an obese individual's risk of heart disease may be modified after maternal NR. Pregnant sheep were fed 100% (control) or 50% (NR) energy requirement from days 30 to 80 of gestation and 100% energy requirement thereafter. At weaning, offspring were exposed to an obesogenic environment or remained lean. At approximately 1 yr of age, the hemodynamic response of these offspring to hypotension, together with left ventricular expression profiles of fatty acid-binding protein 3 (FABP3), peroxisome proliferator-activated receptor-gamma (PPARgamma) and its coactivator (PGC)-1alpha, acetyl-CoA carboxylase (ACC), AMP-activated protein kinase (AMPK)-alpha(2), and voltage-dependent anion channel 1 (VDAC1), was determined. Obesity produced left ventricular hypertrophy in all animals, with increased ectopic (myocardial) lipid in NR offspring. Obesity per se significantly reduced myocardial transcript expression of PGC-1alpha, AMPKalpha(2), VDAC1, and ACC and increased expression of PPARgamma and FABP3. However, although NR animals were similarly obese, their transcript expression of ACC, PPARgamma, and FABP3 was similar to that of lean animals, indicating altered cardiac energy metabolism. Indeed, blunted tachycardia and an amplified inotropic response to hypotension characterized cardiac function in obese NR offspring. The results suggest that maternal NR during early organogenesis can precipitate an altered myocardial response to hypotension and increased myocardial lipid deposition in the adult offspring after adolescent-onset obesity, potentially rendering these individuals more at risk of early heart failure as they age.
Figures



References
-
- Alvarez GE, Beske SD, Ballard TP, Davy KP. Sympathetic neural activation in visceral obesity. Circulation 106: 2533–2536, 2002. - PubMed
-
- Arany Z, Lebrasseur N, Morris C, Smith E, Yang W, Ma Y, Chin S, Spiegelman BM. The transcriptional coactivator PGC-1β drives the formation of oxidative type IIX fibers in skeletal muscle. Cell Metab 5: 35–46, 2007. - PubMed
-
- Banerjee S, Peterson LR. Myocardial metabolism and cardiac performance in obesity and insulin resistance. Curr Cardiol Rep 9: 143–149, 2007. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

