Subunit-selective interrogation of CO recombination in carbonmonoxy hemoglobin by isotope-edited time-resolved resonance Raman spectroscopy
- PMID: 19245215
- PMCID: PMC2722936
- DOI: 10.1021/bi802190f
Subunit-selective interrogation of CO recombination in carbonmonoxy hemoglobin by isotope-edited time-resolved resonance Raman spectroscopy
Abstract
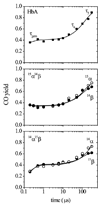
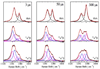
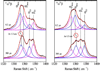
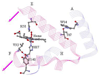
Hemoglobin (Hb) is an allosteric tetrameric protein made up of alphabeta heterodimers. The alpha and beta chains are similar, but are chemically and structurally distinct. To investigate dynamical differences between the chains, we have prepared tetramers in which the chains are isotopically distinguishable, via reconstitution with (15)N-heme. Ligand recombination and heme structural evolution, following HbCO dissociation, was monitored with chain selectivity by resonance Raman (RR) spectroscopy. For alpha but not for beta chains, the frequency of the nu(4) porphyrin breathing mode increased on the microsecond time scale. This increase is a manifestation of proximal tension in the Hb T-state, and its time course is parallel to the formation of T contacts, as determined previously by UVRR spectroscopy. Despite the localization of proximal constraint in the alpha chains, geminate recombination was found to be equally probable in the two chains, with yields of 39 +/- 2%. We discuss the possibility that this equivalence is coincidental, in the sense that it arises from the evolutionary pressure for cooperativity, or that it reflects mechanical coupling across the alphabeta interface, evidence for which has emerged from UVRR studies of site mutants.
Figures



References
-
- Perutz MF. Mechanisms of Cooperativity and Allosteric Regulation in Proteins. Cambridge: Cambridge University Press; 1990. - PubMed
-
- Baldwin J, Chothia C. Hemoglobin - Structural-Changes Related to Ligand-Binding and Its Allosteric Mechanism. J. Mol. Biol. 1979;129 175-&. - PubMed
-
- Monod J, Wyman J, Changeux JP. On nature of allosteric transitions-A plausible model. J. Mol. Biol. 1965;12 88-&. - PubMed
-
- Perutz MF. Stereochemistry of cooperative effects in haemoglobin. Nature. 1970;228 726-&. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

