Multicenter trial of early hypothermia in severe brain injury
- PMID: 19245306
- PMCID: PMC2754260
- DOI: 10.1089/neu.2008.0556
Multicenter trial of early hypothermia in severe brain injury
Abstract
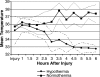
The North American Brain Injury Study: Hypothermia IIR (NABIS:H IIR) is a randomized clinical trial designed to enroll 240 patients with severe brain injury between the ages of 16 and 45 years. The primary outcome measure is the dichotomized Glasgow Outcome Scale (GOS) at 6 months after injury. The study has the power to detect a 17.5% absolute difference in the percentage of patients with a good outcome with a power of 80%. All patients are randomized by waiver of consent unless family is immediately available. Enrollment is within 2.5 h of injury. Patients may be enrolled in the field by emergency medical services personnel affiliated with the study or by study personnel when the patient arrives at the emergency department. Patients who do not follow commands and have no exclusion criteria and who are enrolled in the hypothermia arm of the study are cooled to 35 degrees C as rapidly as possible by intravenous administration of up to 2 liters of chilled crystalloid. Those patients who meet the criteria for the second phase of the protocol (primarily a post-resuscitation GCS 3-8 without hypotension and without severe associated injuries) are cooled to 33 degrees C. Patients enrolled in the normothermia arm receive standard management at normothermia. As of December 2007, 74 patients had been randomized into phase II of the protocol. Patients in the hypothermia arm reached 35 degrees C in 2.7 +/- 1.1 (SD) h after injury and reached 33 degrees C at 4.4 +/- 1.5 h after injury.
Figures
References
-
- Clifton G.L. Miller ER. Choi S.C. Levin H.S. McCauley S. Smith K.R., Jr. Muizelaar J.P. Wagner F.C., Jr. Marion D.W. Luerssen T.G. Chesnut R.M. Schwartz M. Lack of effect of induction of hypothermia after acute brain injury. N. Engl. J. Med. 2001;344:556–563. - PubMed
-
- Clifton G.L. Miller E.R. Choi S.C. Levin H.S. McCauley S. Smith K.R., Jr. Muizelaar J.P. Marion D.W. Luerssen T.G. Hypothermia on admission in patients with severe brain injury. J. Neurotrauma. 2002;19:293–301. - PubMed
-
- Eisenberg H.M. Frankowski R.F. Contant C.F. Marshall L.F. Walker M.D. High-dose barbiturate control of elevated intracranial pressure in patients with severe head injury. J. Neurosurg. 1988;69:15–23. - PubMed
-
- Hutchison J.S. Ward R.E. Lacroix J. Hebert P.C. Barnes M.A. Bohn D.J. Dirks P.B. Doucette S. Fergusson D. Gottesman R. Joffe A.R. Kirpalani H.M. Meyer P.G. Morris K.P. Moher D. Singh R.N. Skippen P.W. Hypothermia therapy after traumatic brain injury in children. N. Engl. J. Med. 2008;358:2447–2456. - PubMed
-
- Markgraf C.G. Clifton G.L. Moody M.R. Treatment window for hypothermia in brain injury. J. Neurosurg. 2001;95:979–983. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials