Ultrastructural evidence of a vesicle-mediated mode of cell degranulation in chicken chromaffin cells during the late phase of embryonic development
- PMID: 19245498
- PMCID: PMC2673782
- DOI: 10.1111/j.1469-7580.2008.01032.x
Ultrastructural evidence of a vesicle-mediated mode of cell degranulation in chicken chromaffin cells during the late phase of embryonic development
Abstract
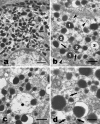
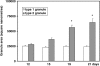
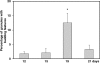
In the present investigation, we attempted to determine whether ultrastructural features indicative of a vesicle-mediated mode of cell secretion were detectable in chick chromaffin cells during embryo development. The adrenal anlagen of domestic fowls were examined at embryonic days (E) 12, 15, 19 and 21 by electron microscopy quantitative analysis. Morphometric evaluation revealed a series of granule and cytoplasmic changes highly specific for piecemeal degranulation (PMD), a secretory process based on vesicular transport of cargoes from within granules for extracellular release. At E19 and E21 we found a significant peak in the percentage of granules exhibiting changes indicative of progressive release of secretory materials, i.e. granules with lucent areas in their cores, reduced electron density, disassembled matrices, residual cores and membrane empty containers. A dramatic raise in the density of 30-80-nm-diameter, membrane-bound, electron-dense and electron-lucent vesicles--which were located either next to granules or close to the plasma membrane--was recognizable at E19, that is, during the prehatching phase. The cytoplasmic burst of dense and clear vesicles was paralleled by the appearance of chromaffin granules showing outpouches or protrusions of their profiles ('budding features'). These ultrastructural data are indicative of an augmented vesicle-mediated transport of chromaffin granule products for extracellular release in chick embryo chromaffin cells during the prehatching stage. In conclusion, this study provides new data on the fine structure of chromaffin cell organelles during organ development and suggests that PMD may be part of an adrenomedullary secretory response that occurs towards the end of chicken embryogenesis. From an evolutionary point of view, this study lends support to the concept that PMD is a secretory mechanism highly conserved throughout vertebrate classes.
Figures




Similar articles
-
Suggestive evidence of a vesicle-mediated mode of cell degranulation in chromaffin cells. A high-resolution scanning electron microscopy investigation.J Anat. 2010 Apr;216(4):518-24. doi: 10.1111/j.1469-7580.2009.01198.x. Epub 2010 Jan 28. J Anat. 2010. PMID: 20136671 Free PMC article.
-
Chromaffin cells in the amphibian urodele Triturus carnifex show ultrastructural features indicative of a vesicle-mediated mode of cell degranulation.Anat Rec (Hoboken). 2009 Jan;292(1):73-8. doi: 10.1002/ar.20749. Anat Rec (Hoboken). 2009. PMID: 18727112
-
Chromaffin cells in the adrenal homolog of Aphanius fasciatus (teleost fish) express piecemeal degranulation in response to osmotic stress: a hint for a conservative evolutionary process.Anat Rec A Discov Mol Cell Evol Biol. 2006 Oct;288(10):1077-86. doi: 10.1002/ar.a.20372. Anat Rec A Discov Mol Cell Evol Biol. 2006. PMID: 16964607
-
Cell secretion mediated by granule-associated vesicle transport: a glimpse at evolution.Anat Rec (Hoboken). 2010 Jul;293(7):1115-24. doi: 10.1002/ar.21146. Anat Rec (Hoboken). 2010. PMID: 20340095 Review.
-
Dense-core granules in neuroendocrine cells and neurons release their secretory constituents by piecemeal degranulation (review).Int J Mol Med. 2006 Dec;18(6):1037-46. Int J Mol Med. 2006. PMID: 17089006 Review.
Cited by
-
Suggestive evidence of a vesicle-mediated mode of cell degranulation in chromaffin cells. A high-resolution scanning electron microscopy investigation.J Anat. 2010 Apr;216(4):518-24. doi: 10.1111/j.1469-7580.2009.01198.x. Epub 2010 Jan 28. J Anat. 2010. PMID: 20136671 Free PMC article.
References
-
- Anderson DJ. Molecular control of cell fate in the neural crest: the sympathoadrenal lineage. Annu Rev Neurosci. 1993;16:129–158. - PubMed
-
- Bouceck RJ, Bourne B. Catecholamines of the allantoic fluid in the developing chick embryo. Nature. 1962;193:1181–1182. - PubMed
-
- Bournaud R, Hidalgo J, Yu H, Girard E, Shimahara T. Catecholamine secretion from rat foetal adrenal chromaffin cells and hypoxia sensitivity. Pflugers Arch – Eur J Physiol. 2007;454:83–92. - PubMed
-
- Cheung CY. Fetal adrenal medulla catecholamine response to hypoxia – direct and neural components. Am J Physiol. 1990;258:R1340–1346. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

