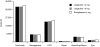Long-term outcomes in patients with type 2 diabetes receiving glimepiride combined with liraglutide or rosiglitazone
- PMID: 19245711
- PMCID: PMC2667489
- DOI: 10.1186/1475-2840-8-12
Long-term outcomes in patients with type 2 diabetes receiving glimepiride combined with liraglutide or rosiglitazone
Abstract
Background: Poor control of type 2 diabetes results in substantial long-term consequences. Studies of new diabetes treatments are rarely designed to assess mortality, complication rates and costs. We sought to estimate the long-term consequences of liraglutide and rosiglitazone both added to glimepiride.
Methods: To estimate long-term clinical and economic consequences, we used the CORE diabetes model, a validated cohort model that uses epidemiologic data from long-term clinical trials to simulate morbidity, mortality and costs of diabetes. Clinical data were extracted from the LEAD-1 trial evaluating two doses (1.2 mg and 1.8 mg) of a once daily GLP-1 analog liraglutide, or rosiglitazone 4 mg, on a background of glimepiride in type 2 diabetes. CORE was calibrated to the LEAD-1 baseline patient characteristics. Survival, cumulative incidence of cardiovascular, ocular and renal events and healthcare costs were estimated over three periods: 10, 20 and 30 years.
Results: In a hypothetical cohort of 5000 patients per treatment followed for 30 years, liraglutide 1.2 mg and 1.8 mg had higher survival rates compared to the group treated with rosiglitazone (15.0% and 16.0% vs. 12.6% after 30 years), and fewer cardiovascular, renal, and ocular events. Cardiovascular death rates after 30 years were 69.7%, 68.4% and 72.5%, for liraglutide 1.2 mg, 1.8 mg, and rosiglitazone, respectively. First and recurrent amputations were lower in the rosiglitazone group, probably due to a 'survival paradox' in the liraglutide arms (number of events: 565, 529, and 507, respectively). Overall cumulative costs per patient, were lower in both liraglutide groups compared to rosiglitazone (US$38,963, $39,239, and $40,401 for liraglutide 1.2 mg, 1.8 mg, and rosiglitazone, respectively), mainly driven by the costs of cardiovascular events in all groups.
Conclusion: Using data from LEAD-1 and epidemiologic evidence from the CORE diabetes model, projected rates of mortality, diabetes complications and healthcare costs over the long term favor liraglutide plus glimepiride over rosiglitazone plus glimepiride.
Trial registration: LEAD-1 NCT00318422; LEAD-2 NCT00318461; LEAD-3 NCT 00294723; LEAD-4 NCT00333151; LEAD-5 NCT00331851; LEAD-6 NCT00518882.
Trial registration: ClinicalTrials.gov NCT00318422 NCT00318461 NCT00331851 NCT00333151 NCT00518882 NCT00294723.
Figures
References
-
- UKPDS Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837–853. doi: 10.1016/S0140-6736(98)07019-6. - DOI - PubMed
-
- World Health Organization 2008. http://www.who.int/mediacentre/factsheets/fs312/en/
-
- CDC National Center for Chronic Disease Prevention and Health Promotion 2007 National Diabetes Fact Sheet http://www.cdc.gov/diabetes/pubs/factsheet07.htm
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical