Mechanisms contributing to synaptic Ca2+ signals and their heterogeneity in hair cells
- PMID: 19246382
- PMCID: PMC2657422
- DOI: 10.1073/pnas.0813213106
Mechanisms contributing to synaptic Ca2+ signals and their heterogeneity in hair cells
Abstract
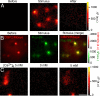
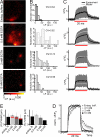
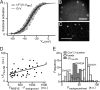
Sound coding at hair cell ribbon synapses is tightly regulated by Ca(2+). Here, we used patch-clamp, fast confocal Ca(2+) imaging and modeling to characterize synaptic Ca(2+) signaling in cochlear inner hair cells (IHCs) of hearing mice. Submicrometer fluorescence hotspots built up and collapsed at the base of IHCs within a few milliseconds of stimulus onset and cessation. They most likely represented Ca(2+) microdomains arising from synaptic Ca(2+) influx through Ca(V)1.3 channels. Synaptic Ca(2+) microdomains varied substantially in amplitude and voltage dependence even within single IHCs. Testing putative mechanisms for the heterogeneity of Ca(2+) signaling, we found the amplitude variability unchanged when blocking mitochondrial Ca(2+) uptake or Ca(2+)-induced Ca(2+) release, buffering cytosolic Ca(2+) by millimolar concentrations of EGTA, or elevating the Ca(2+) channel open probability by the dihydropyridine agonist BayK8644. However, we observed substantial variability also for the fluorescence of immunolabeled Ca(V)1.3 Ca(2+) channel clusters. Moreover, the Ca(2+) microdomain amplitude correlated positively with the size of the corresponding synaptic ribbon. Ribbon size, previously suggested to scale with the number of synaptic Ca(2+) channels, was approximated by using fluorescent peptide labeling. We propose that IHCs adjust the number and the gating of Ca(V)1.3 channels at their active zones to diversify their transmitter release rates.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Fuchs PA, Glowatzki E, Moser T. The afferent synapse of cochlear hair cells. Curr Opin Neurobiol. 2003;13:452–458. - PubMed
-
- Parsons TD, Lenzi D, Almers W, Roberts WM. Calcium-triggered exocytosis and endocytosis in an isolated presynaptic cell: Capacitance measurements in saccular hair cells. Neuron. 1994;13:875–883. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

