A retrograde signal from RyR1 alters DHP receptor inactivation and limits window Ca2+ release in muscle fibers of Y522S RyR1 knock-in mice
- PMID: 19246389
- PMCID: PMC2657461
- DOI: 10.1073/pnas.0812661106
A retrograde signal from RyR1 alters DHP receptor inactivation and limits window Ca2+ release in muscle fibers of Y522S RyR1 knock-in mice
Abstract
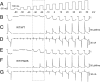
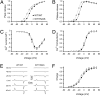
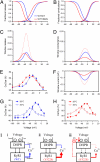
Malignant hyperthermia (MH) is a life-threatening hypermetabolic condition caused by dysfunctional Ca(2+) homeostasis in skeletal muscle, which primarily originates from genetic alterations in the Ca(2+) release channel (ryanodine receptor, RyR1) of the sarcoplasmic reticulum (SR). Owing to its physical interaction with the dihydropyridine receptor (DHPR), RyR1 is controlled by the electrical potential across the transverse tubular (TT) membrane. The DHPR exhibits both voltage-dependent activation and inactivation. Here we determined the impact of an MH mutation in RyR1 (Y522S) on these processes in adult muscle fibers isolated from heterozygous RyR1(Y522S)-knock-in mice. The voltage dependence of DHPR-triggered Ca(2+) release flux was left-shifted by approximately 8 mV. As a consequence, the voltage window for steady-state Ca(2+) release extended to more negative holding potentials in muscle fibers of the RyR1(Y522S)-mice. A rise in temperature from 20 degrees to 30 degrees C caused a further shift to more negative potentials of this window (by approximately 20 mV). The activation of the DHPR-mediated Ca(2+) current was minimally changed by the mutation. However, surprisingly, the voltage dependence of steady-state inactivation of DHPR-mediated calcium conductance and release were also shifted by approximately 10 mV to more negative potentials, indicating a retrograde action of the RyR1 mutation on DHPR inactivation that limits window Ca(2+) release. This effect serves as a compensatory response to the lowered voltage threshold for Ca(2+) release caused by the Y522S mutation and represents a novel mechanism to counteract excessive Ca(2+) leak and store depletion in MH-susceptible muscle.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
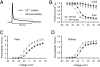

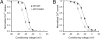
References
-
- MacLennan DH, Phillips MS. Malignant hyperthermia. Science. 1992;256:789–794. - PubMed
-
- Lehmann-Horn F, Jurkat-Rott K. Voltage-gated ion channels and hereditary disease. Physiol Rev. 1999;79:1317–1372. - PubMed
-
- Robinson R, Carpenter D, Shaw MA, Halsall J, Hopkins P. Mutations in RYR1 in malignant hyperthermia and central core disease. Hum Mutat. 2006;27:977–989. - PubMed
-
- Melzer W, Herrmann-Frank A, Lüttgau HC. The role of Ca2+ ions in excitation-contraction coupling of skeletal muscle fibres. Biochim Biophys Acta. 1995;1241:59–116. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

