RE-1-silencing transcription factor shows tumor-suppressor functions and negatively regulates the oncogenic TAC1 in breast cancer cells
- PMID: 19246391
- PMCID: PMC2647978
- DOI: 10.1073/pnas.0809130106
RE-1-silencing transcription factor shows tumor-suppressor functions and negatively regulates the oncogenic TAC1 in breast cancer cells
Abstract
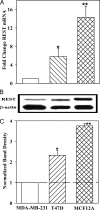
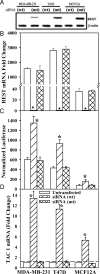
Breast cancer remains the most prevalent cancer among women in the United States. Substance P, a peptide derived from the TAC1 gene, mediates oncogenic properties in breast and other cancers. TAC1 expression facilitates the entry of breast cancer cells into bone marrow. The transcriptional repressor element 1-silencing transcription factor (REST) has been implicated in both oncogenic and tumor-suppressor functions. REST binds to the 5' untranslated region of the TAC1 promoter and suppresses its expression. This study investigated a role for REST in TAC1 induction in breast cancer. Western blots and real-time PCR indicated that REST expression in breast cancer cells was inversely proportional to the cells' aggressiveness, for both cell lines and primary breast cancer cells. REST knockdown in low-metastatic T47D cells and nontumorigenic MCF12A cells resulted in increases in TAC1 induction, proliferation, and migration. These parameters were negatively affected by ectopic expression of REST in highly aggressive MDA-MB-231 cells. Together, these findings show a central role for REST in the oncogenic function of TAC1 and suggest a tumor-suppressor role for REST in breast cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Jemal A, et al. Cancer Statistics, 2003. CA Cancer J Clin. 2003;53:5–26. - PubMed
-
- Rao G, et al. Facilitating role of preprotachykinin-I gene in the integration of breast cancer cells within the stromal compartment of the bone marrow: A model of early cancer progression. Cancer Res. 2004;64:2874–2881. - PubMed
-
- Rameshwar P, Oh HS, Yook C, Gascon P, Chang VT. Substance p-fibronectin-cytokine interactions in myeloproliferative disorders with bone marrow fibrosis. Acta Haematol. 2003;109:1–10. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

