A highly conserved motif within the NH2-terminal coiled-coil domain of angiopoietin-like protein 4 confers its inhibitory effects on lipoprotein lipase by disrupting the enzyme dimerization
- PMID: 19246456
- PMCID: PMC2673263
- DOI: 10.1074/jbc.M809802200
A highly conserved motif within the NH2-terminal coiled-coil domain of angiopoietin-like protein 4 confers its inhibitory effects on lipoprotein lipase by disrupting the enzyme dimerization
Abstract
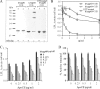
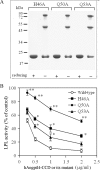
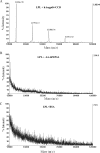
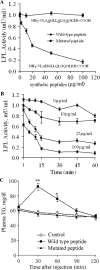
Lipoprotein lipase (LPL) is a principal enzyme responsible for the clearance of chylomicrons and very low density lipoproteins from the bloodstream. Two members of the Angptl (angiopoietin-like protein) family, namely Angptl3 and Angptl4, have been shown to inhibit LPL activity in vitro and in vivo. Here, we further investigated the structural basis underlying the LPL inhibition by Angptl3 and Angptl4. By multiple sequence alignment analysis, we have identified a highly conserved 12-amino acid consensus motif that is present within the coiled-coil domain (CCD) of both Angptl3 and Angptl4, but not other members of the Angptl family. Substitution of the three polar amino acid residues (His(46), Gln(50), and Gln(53)) within this motif with alanine abolishes the inhibitory effect of Angptl4 on LPL in vitro and also abrogates the ability of Angptl4 to elevate plasma triglyceride levels in mice. The CCD of Angptl4 interacts with LPL and converts the catalytically active dimers of LPL to its inactive monomers, whereas the mutant protein with the three polar amino acids being replaced by alanine loses such a property. Furthermore, a synthetic peptide consisting of the 12-amino acid consensus motif is sufficient to inhibit LPL activity, although the potency is much lower than the recombinant CCD of Angptl4. In summary, our data suggest that the 12-amino acid consensus motif within the CCD of Angptl4, especially the three polar residues within this motif, is responsible for its interaction with and inhibition of LPL by blocking the enzyme dimerization.
Figures



References
-
- Mead, J. R., Irvine, S. A., and Ramji, D. P. (2002) J. Mol. Med. 80 753–769 - PubMed
-
- Merkel, M., Eckel, R. H., and Goldberg, I. J. (2002) J. Lipid Res. 43 1997–2006 - PubMed
-
- Otarod, J. K., and Goldberg, I. J. (2004) Curr. Atheroscler. Rep. 6 335–342 - PubMed
-
- Enerback, S., Semb, H., Tavernier, J., Bjursell, G., and Olivecrona, T. (1988) Gene (Amst.) 64 97–106 - PubMed
-
- Doolittle, M. H., Ben-Zeev, O., Elovson, J., Martin, D., and Kirchgessner, T. G. (1990) J. Biol. Chem. 265 4570–4577 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

