Gamma-synucleinopathy: neurodegeneration associated with overexpression of the mouse protein
- PMID: 19246516
- PMCID: PMC2671987
- DOI: 10.1093/hmg/ddp090
Gamma-synucleinopathy: neurodegeneration associated with overexpression of the mouse protein
Abstract
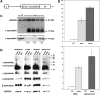
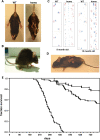
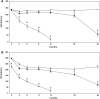
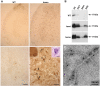
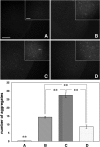
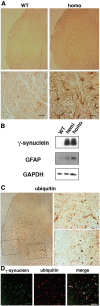
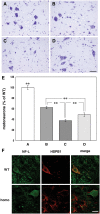
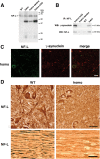
The role of alpha-synuclein in pathogenesis of familial and idiopathic forms of Parkinson's disease, and other human disorders known as alpha-synucleinopathies, is well established. In contrast, the involvement of two other members of the synuclein family, beta-synuclein and gamma-synuclein, in the development and progression of neurodegeneration is poorly studied. However, there is a growing body of evidence that alpha-synuclein and beta-synuclein have opposite neuropathophysiological effects. Unlike alpha-synuclein, overexpressed beta-synuclein does not cause pathological changes in the nervous system of transgenic mice and even ameliorates the pathology caused by overexpressed alpha-synuclein. To assess the consequences of excess expression of the third family member, gamma-synuclein, on the nervous system we generated transgenic mice expressing high levels of mouse gamma-synuclein under control of Thy-1 promoter. These animals develop severe age- and transgene dose-dependent neuropathology, motor deficits and die prematurely. Histopathological changes include aggregation of gamma-synuclein, accumulation of various inclusions in neuronal cell bodies and processes, and astrogliosis. These changes are seen throughout the nervous system but are most prominent in the spinal cord where they lead to loss of spinal motor neurons. Our data suggest that down-regulation of small heat shock protein HSPB1 and disintegration of neurofilament network play a role in motor neurons dysfunction and death. These findings demonstrate that gamma-synuclein can be involved in neuropathophysiological changes and the death of susceptible neurons suggesting the necessity of further investigations of the potential role of this synuclein in disease.
Figures
References
-
- Park J.Y., Lansbury P.T., Jr Beta-synuclein inhibits formation of alpha-synuclein protofibrils: a possible therapeutic strategy against Parkinson’s disease. Biochemistry. 2003;42:3696–3700. - PubMed
-
- da Costa C.A., Masliah E., Checler F. Beta-synuclein displays an antiapoptotic p53-dependent phenotype and protects neurons from 6-hydroxydopamine-induced caspase 3 activation: cross-talk with alpha-synuclein and implication for Parkinson’s disease. J. Biol. Chem. 2003;278:37330–37335. - PubMed
-
- Windisch M., Hutter-Paier B., Rockenstein E., Hashimoto M., Mallory M., Masliah E. Development of a new treatment for Alzheimer’s disease and Parkinson’s disease using anti-aggregatory beta-synuclein-derived peptides. J. Mol. Neurosci. 2002;19:63–69. - PubMed
-
- Hashimoto M., Rockenstein E., Mante M., Mallory M., Masliah E. β-Synuclein inhibits alpha-synuclein aggregation: a possible role as an anti-parkinsonian factor. Neuron. 2001;32:213–223. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

