NtCP56, a new cysteine protease in Nicotiana tabacum L., involved in pollen grain development
- PMID: 19246592
- PMCID: PMC2671612
- DOI: 10.1093/jxb/erp022
NtCP56, a new cysteine protease in Nicotiana tabacum L., involved in pollen grain development
Abstract
Proteinases play a critical role in developmental homeostasis and in response to environ-mental stimuli. Our present research reports that a new cysteine protease, NtCP56, is involved in the development of pollen grains in Nicotiana tabacum L. The NtCP56 gene, which encodes a protein of 361 amino acid residues with a calculated molecular mass of 40 kDa, is strongly expressed in anthers. The recombinant NtCP56 showed a high activity towards casein. Kinetic analysis revealed a K(m) of 2.20 mg ml(-1) and V(max) of 11.07 microg ml(-1) min(-1). The recombinant NtCP56 retained more than 50% of its maximum enzymatic activity from 20 degrees C to 60 degrees C with an optimum Tm range of 30-50 degrees C. The enzyme had a maximum activity at approximately pH 6.5. Suppression of the NtCP56 gene in anti-sense transgenic tobaccos resulted in the sterility of pollen grains. Our data indicated that, as a cysteine protease, NtCP56 might play an important role in pollen development.
Figures




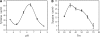


References
-
- Beyene G, Foyer CH, Kunert KJ. Two new cysteine proteinases with specific expression patterns in mature and senescent tobacco (Nicotiana tabacum L.) leaves. Journal of Experimental Botany. 2006;57:1431–1443. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

