Large CTG repeats trigger p16-dependent premature senescence in myotonic dystrophy type 1 muscle precursor cells
- PMID: 19246640
- PMCID: PMC2671374
- DOI: 10.2353/ajpath.2009.080560
Large CTG repeats trigger p16-dependent premature senescence in myotonic dystrophy type 1 muscle precursor cells
Abstract
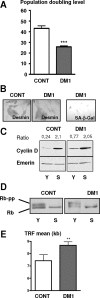
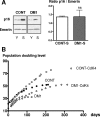
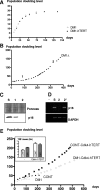
A CTG repeat amplification is responsible for the dominantly inherited neuromuscular disorder, myotonic dystrophy type 1 (DM1), which is characterized by progressive muscle wasting and weakness. The expanded (CTG)n tract not only alters the myogenic differentiation of the DM1 muscle precursor cells but also reduces their proliferative capacity. In this report, we show that these muscle precursor cells containing large CTG expansion sequences have not exhausted their proliferative capacity, but have entered into premature senescence. We demonstrate that an abnormal accumulation of p16 is responsible for this defect because the abolition of p16 activity overcomes early growth arrest and restores an extended proliferative capacity. Our results suggest that the accelerated telomere shortening measured in DM1 cells does not contribute to the aberrant induction of p16. We propose that a cellular stress related to the amplified CTG repeat promotes premature senescence mediated by a p16-dependent pathway in DM1 muscle precursor cells. This mechanism is responsible for the reduced proliferative capacity of the DM1 muscle precursor cells and could participate in both the impaired regeneration and atrophy observed in the DM1 muscles containing large CTG expansions.
Figures

References
-
- Harper PS. London: W.B. Saunders,; Myotonic Dystrophy. (ed 3) 2004:pp. 17–45.
-
- Aslanidis C, Jansen G, Amemiya C, Shutler G, Mahadevan M, Tsilfidis C, Chen C, Alleman J, Wormskamp NG, Vooijs M, Buxton J, Johnson K, Smeets JM, Lennon G, Carrano AV, Korneluk R, Wieringa B, de Jong P. Cloning of the essential myotonic dystrophy region and mapping of the putative defect. Nature. 1992;355:548–551. - PubMed
-
- Brook JD, McCurrach ME, Harley HG, Buckler AJ, Church D, Aburatani H, Hunter K, Stanton VP, Thirion JP, Hudson T, Sohn R, Zemelman B, Snell RG, Rundle SA, Crow S, Davies J, Shelbourne P, Buxton J, Jones C, Juvonen V, Johnson K, Harper P, Shaw DJ, Housman D. Molecular basis of myotonic dystrophy: expansion of a trinucleotide (CTG) repeat at the 3′ end of a transcript encoding a protein kinase family member. Cell. 1992;68:799–808. - PubMed
-
- Fu YH, Pizzuti A, Fenwick RG, Jr, King J, Rajnarayan S, Dunne PW, Dubel J, Nasser GA, Ashizawa T, de Jong P, Wieringa B, Korneluk R, Perryman MB, Epstein HF, Caskey CT. An unstable triplet repeat in a gene related to myotonic muscular dystrophy. Science. 1992;255:1256–1258. - PubMed
-
- Mahadevan M, Tsilfidis C, Sabourin L, Shutler G, Amemiya C, Jansen G, Neville C, Narang M, Barcelo J, O'Hoy K, Leblond S, Earl-Macdonald J, de Jong P, Wieringa B, Korneluk R. Myotonic dystrophy mutation: an unstable CTG repeat in the 3′ untranslated region of the gene. Science. 1992;255:1253–1255. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

